Horizontal Columns On The Periodic Table Are Called
listenit
Mar 22, 2025 · 8 min read
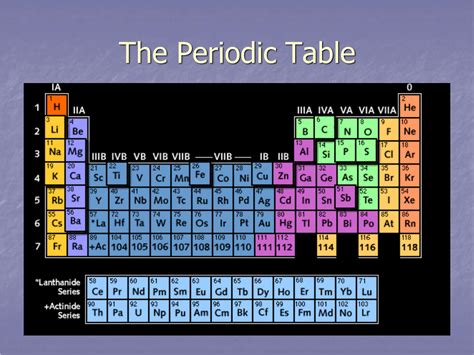
Table of Contents
Horizontal Columns on the Periodic Table are Called: Periods – A Deep Dive into Periodic Trends
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and recurring chemical properties. Understanding its organization is crucial to grasping the fundamentals of chemistry. One of the most basic yet important aspects of the periodic table's structure is the arrangement of elements into horizontal rows called periods. This article delves deep into what periods are, what they represent, and how they relate to periodic trends in elemental properties.
What are Periods in the Periodic Table?
The horizontal rows in the periodic table are known as periods. Each period represents an energy level or electron shell in an atom. As you move across a period from left to right, you add one proton and one electron to the atom. This increase in atomic number directly affects the properties of the elements within that period. The number of elements in each period is not constant; it varies depending on the number of orbitals available at that particular energy level.
Significance of Period Number
The period number corresponds to the highest principal quantum number (n) of the electrons in the ground state of the atoms of that period. For instance, elements in Period 1 have electrons only in the n=1 energy level, while elements in Period 2 have electrons in both n=1 and n=2 energy levels. This simple relationship directly links the period number to the electronic configuration and, subsequently, the chemical properties of the elements.
Periodic Trends and Periods
The periodic table's structure beautifully illustrates periodic trends – the gradual changes in elemental properties as you move across a period or down a group. These trends are directly linked to the electron configuration and the effective nuclear charge experienced by the outermost electrons (valence electrons).
Atomic Radius
Moving across a period from left to right, the atomic radius generally decreases. This occurs because the number of protons in the nucleus increases, resulting in a stronger positive charge attracting the electrons more tightly. While additional electrons are added, they are placed in the same energy level, and the increased nuclear charge outweighs the shielding effect provided by the inner electrons.
Ionization Energy
Ionization energy, the energy required to remove an electron from an atom, generally increases across a period. This is a direct consequence of the decreasing atomic radius. The stronger attraction between the nucleus and electrons makes it harder to remove an electron, hence the higher ionization energy.
Electronegativity
Electronegativity, the ability of an atom to attract electrons in a chemical bond, generally increases across a period. Like ionization energy, this trend is a consequence of the increasing nuclear charge and decreasing atomic radius. Atoms with higher electronegativity have a stronger pull on shared electrons in a bond.
Electron Affinity
Electron affinity, the energy change when an atom gains an electron, generally shows a more complex trend across a period. While there's a general tendency for it to increase, there are exceptions due to the electronic configuration and stability of the half-filled and completely filled subshells.
Metallic Character
The metallic character of elements generally decreases across a period. This means that elements on the left side of a period tend to be more metallic (e.g., showing properties like good electrical conductivity, malleability, and ductility), while those on the right side tend to be more non-metallic (e.g., showing properties like poor electrical conductivity and brittleness). This trend is directly related to ionization energy and electronegativity; metals tend to have lower ionization energies and electronegativities than non-metals.
Periods and Electron Shells
The connection between periods and electron shells is fundamental to understanding the periodic table's organization. Each period corresponds to the filling of a principal electron shell:
- Period 1: Fills the 1s subshell (containing a maximum of 2 electrons).
- Period 2: Fills the 2s and 2p subshells (containing a maximum of 8 electrons).
- Period 3: Fills the 3s and 3p subshells (containing a maximum of 8 electrons).
- Period 4: Fills the 4s, 3d, and 4p subshells (containing a maximum of 18 electrons).
- Period 5: Fills the 5s, 4d, and 5p subshells (containing a maximum of 18 electrons).
- Period 6: Fills the 6s, 4f, 5d, and 6p subshells (containing a maximum of 32 electrons).
- Period 7: Fills the 7s, 5f, 6d, and 7p subshells (partially filled).
The gradual filling of these subshells dictates the number of elements in each period and significantly influences their chemical and physical properties. The inclusion of the d-block elements (transition metals) in Periods 4-7 and the f-block elements (lanthanides and actinides) in Periods 6 and 7 adds complexity and expands the number of elements within those periods.
Exceptions to Periodic Trends
While the general trends discussed above are generally observed, there are always exceptions. These exceptions often arise due to:
- Electron-electron repulsions: The increased repulsion between electrons in the same subshell can slightly alter the expected trend.
- Shielding effects: Inner electrons shield the outer electrons from the full positive nuclear charge. Variations in shielding effects can influence atomic radii and ionization energies.
- Half-filled and fully filled subshells: Atoms with half-filled or fully filled subshells exhibit extra stability. This increased stability can result in slight deviations from the anticipated trends in ionization energy and electron affinity.
These exceptions demonstrate the complex interplay of factors that determine the properties of elements. Understanding these exceptions is crucial for a comprehensive understanding of the periodic table and its implications.
The Importance of Understanding Periods
Understanding the concept of periods is fundamental to many aspects of chemistry:
- Predicting properties: Knowing an element's period helps predict its chemical and physical properties based on periodic trends.
- Explaining reactivity: The electron configuration associated with an element's period explains its reactivity and how it will interact with other elements.
- Designing new materials: Understanding periodic trends allows scientists to design new materials with specific properties.
- Interpreting chemical reactions: The periodic table and its periods are crucial in understanding and predicting the outcome of chemical reactions.
By understanding the organization of elements into periods and how this relates to electronic configuration and periodic trends, we can unravel the mysteries of the atomic world and predict the behavior of matter.
Beyond the Basics: A Deeper Look at Periodicity
The periodic table is more than just a neat arrangement of elements; it represents a fundamental law of nature – the periodicity of properties. This periodicity arises from the quantum mechanical behavior of electrons within atoms. The repeating patterns in electron configuration across periods directly translate into predictable changes in properties like atomic radius, ionization energy, and electronegativity.
The Quantum Mechanical Basis of Periodicity
The underlying reason for the periodic recurrence of properties is the quantized nature of energy levels in atoms. Electrons occupy specific energy levels or shells, and the filling of these shells follows a specific order dictated by the Aufbau principle and Hund's rule. The maximum number of electrons that can occupy each shell is limited, leading to the repeating patterns observed in the periodic table. When a shell is filled, the next electron must occupy a new, higher energy level, initiating a new period.
Predicting Properties Using Periods and Groups
The combination of period and group information provides a powerful tool for predicting the properties of an element. The period indicates the highest principal quantum number, providing insights into the size and energy levels of the atom. The group number provides information about the number of valence electrons, which are primarily responsible for an element's chemical reactivity. By combining this information, we can make accurate predictions about an element's reactivity, bonding behavior, and other properties.
Applications of Periodic Trends
The understanding of periodic trends has numerous applications in various fields:
- Material science: The design of new materials with specific properties, such as high strength, conductivity, or reactivity, relies heavily on the understanding of periodic trends.
- Catalysis: The choice of catalysts for chemical reactions often hinges on their electronic structure and properties, which are predictable based on their position in the periodic table.
- Medicine: The biological activity of many drugs and other compounds is directly related to their chemical properties, which are largely determined by their position in the periodic table.
- Environmental science: The understanding of environmental processes, such as pollution and remediation, often involves understanding the chemical behavior of elements and their interactions with other substances.
Conclusion: The Enduring Power of the Periodic Table
The horizontal rows in the periodic table, the periods, are more than just a convenient arrangement. They represent a fundamental principle governing the behavior of matter: the periodicity of properties. Understanding the significance of periods, their relationship to electron shells, and the resulting periodic trends is essential for comprehending the fundamental principles of chemistry and applying this knowledge across a wide range of scientific and technological fields. The periodic table, with its periods and groups, remains a powerful tool for organizing and understanding the vast world of chemical elements and their behavior. Its enduring power lies in its ability to predict, explain, and inspire advancements in various scientific domains.
Latest Posts
Latest Posts
-
The Two Dna Strands Are Held Together By
Mar 23, 2025
-
Solving Systems Of Equations By Substitution Answer Key
Mar 23, 2025
-
What Is 5 7 As A Decimal
Mar 23, 2025
-
What Is The Electron Configuration For Arsenic
Mar 23, 2025
-
What Is 33 And 1 3 As A Decimal
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Horizontal Columns On The Periodic Table Are Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
