What Is The Electron Configuration For Arsenic
listenit
Mar 23, 2025 · 5 min read
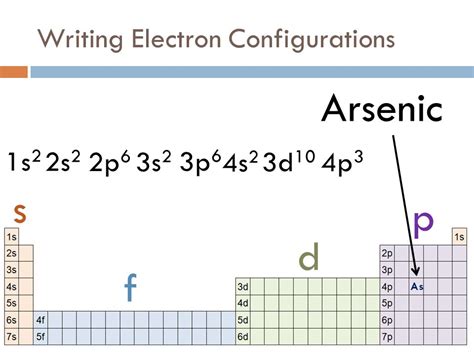
Table of Contents
What is the Electron Configuration for Arsenic? A Deep Dive into Atomic Structure
Arsenic, a metalloid with the symbol As and atomic number 33, holds a fascinating position in the periodic table. Understanding its electron configuration is key to unlocking its unique properties and chemical behavior. This comprehensive guide will explore the electron configuration of arsenic, delve into the principles behind it, and examine its implications for arsenic's role in various fields.
Understanding Electron Configuration
Before we dive into arsenic's specific electron configuration, let's establish a foundational understanding of this concept. Electron configuration describes the arrangement of electrons in an atom's electron shells and subshells. It's governed by the Aufbau principle, which dictates that electrons fill the lowest energy levels first, and the Pauli exclusion principle, which states that no two electrons in an atom can have the same set of four quantum numbers (n, l, ml, and ms). Finally, Hund's rule states that electrons will individually occupy each orbital within a subshell before doubling up.
These principles help us predict the most stable electron arrangement for any element. The electron configuration is often represented using a notation that specifies the principal quantum number (n), the subshell (s, p, d, or f), and the number of electrons in each subshell. For example, 1s² indicates two electrons in the 1s subshell.
Determining the Electron Configuration of Arsenic (As)
Arsenic has an atomic number of 33, meaning it has 33 protons and 33 electrons in its neutral state. To determine its electron configuration, we systematically fill the electron shells and subshells according to the Aufbau principle and Hund's rule.
The order of filling is generally: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p... However, exceptions exist, especially with transition metals and some other elements. Arsenic, fortunately, follows the general trend.
Therefore, the complete electron configuration of arsenic is: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p³.
This can also be written in a condensed or shorthand notation using the noble gas configuration. The noble gas preceding arsenic is Argon (Ar), which has the electron configuration 1s² 2s² 2p⁶ 3s² 3p⁶. Therefore, the condensed electron configuration of arsenic is: [Ar] 3d¹⁰ 4s² 4p³.
This condensed notation simplifies the representation while still conveying all the essential information. It highlights that the inner electrons are arranged like Argon, with the additional electrons occupying the 3d and 4p subshells.
Analyzing Arsenic's Electron Configuration
Let's break down Arsenic's electron configuration to understand its implications:
-
[Ar]: This core configuration represents the stable electron arrangement of Argon, a noble gas. These inner electrons are tightly bound to the nucleus and generally do not participate in chemical bonding.
-
3d¹⁰: The fully filled 3d subshell contributes to arsenic's metallic character. The ten electrons in this subshell are relatively stable, influencing arsenic's physical and chemical properties.
-
4s²: The two electrons in the 4s subshell are also relatively stable, adding to the overall electron distribution.
-
4p³: This partially filled 4p subshell is crucial in determining arsenic's chemical reactivity and bonding behavior. The three unpaired electrons make arsenic relatively reactive, readily forming covalent bonds with other elements. This explains why arsenic forms compounds with a wide range of elements.
Valence Electrons and Chemical Bonding
The valence electrons are the outermost electrons involved in chemical bonding. In arsenic's case, the valence electrons are those in the 4s and 4p subshells, totaling five electrons (4s² 4p³). This explains arsenic's ability to form a variety of compounds with different oxidation states, ranging from -3 to +5.
The presence of three unpaired electrons in the 4p subshell accounts for arsenic's tendency to form covalent bonds, often sharing electrons with other atoms to achieve a more stable electron configuration. This is typical of metalloids, exhibiting properties of both metals and nonmetals.
Arsenic's Properties and Applications
The electron configuration directly influences the various properties and applications of arsenic:
-
Semiconductor Properties: The partially filled 4p subshell contributes to arsenic's semiconductor properties. This means its electrical conductivity can be controlled, making it useful in the electronics industry, notably in the production of semiconductors and certain types of transistors.
-
Toxicity: Arsenic's reactivity, stemming from its unpaired valence electrons, is responsible for its toxicity. The ability to form strong bonds with biological molecules can disrupt cellular processes, leading to harmful effects on living organisms. This is why arsenic compounds are considered hazardous materials requiring careful handling and disposal.
-
Allotropes: Arsenic exists in several allotropic forms, each with slightly different properties. This diversity is partly due to variations in how the valence electrons interact to form different crystal structures.
-
Medicinal Uses (Historically): Despite its toxicity, arsenic has historically been used in small doses for medicinal purposes, although this practice is largely obsolete due to the safer alternatives available. The precise mechanisms of its historical medicinal action relate to its ability to interact with biological systems, but this needs to be handled with extreme caution given its high toxicity.
-
Alloying Agent: Arsenic can be used as an alloying agent in certain metals, modifying their properties to suit specific applications. The ability to form metallic bonds with other elements contributes to its alloying capabilities.
-
Pesticides: Arsenic compounds have been used as pesticides in the past, but due to environmental and health concerns, their use has been significantly restricted. The high reactivity that makes arsenic effective as a pesticide is also the reason for its toxicity.
Conclusion: The Importance of Electron Configuration
The electron configuration of arsenic, [Ar] 3d¹⁰ 4s² 4p³, is fundamental to understanding its properties and behavior. The presence of five valence electrons, specifically the three unpaired electrons in the 4p subshell, explains arsenic's reactivity, ability to form covalent bonds, semiconductor properties, and its toxicity. This understanding is critical in various fields, from material science and electronics to environmental science and toxicology. While arsenic's toxicity necessitates caution, its unique properties, derived directly from its electron configuration, still find applications in specific industrial processes. Further research and development may unlock even more of arsenic's potential, while minimizing its risks. This deep dive into arsenic's electron configuration illustrates the power of understanding atomic structure to explain the macroscopic properties and applications of elements.
Latest Posts
Latest Posts
-
What Is The Difference Between The Plot And The Theme
Mar 25, 2025
-
Ground State Electron Configuration For Calcium
Mar 25, 2025
-
3x To The Power Of 2
Mar 25, 2025
-
What Is The Remainder In The Synthetic Division Problem
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration For Arsenic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
