Group 1a Elements Are Also Called
listenit
Mar 21, 2025 · 6 min read
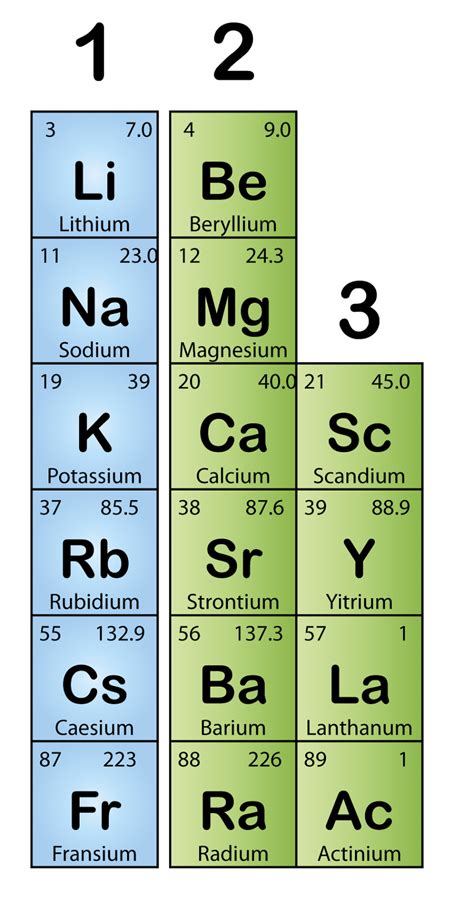
Table of Contents
Group 1A Elements: Also Known as Alkali Metals – A Deep Dive
Group 1A elements, also known as alkali metals, are a fascinating group of chemical elements that share unique properties and exhibit intriguing reactivity. Understanding their characteristics is crucial for various fields, from industrial applications to biological processes. This comprehensive article delves deep into the world of alkali metals, exploring their properties, reactions, uses, and significance.
Defining the Alkali Metals: Properties and Characteristics
The alkali metals encompass the elements located in the first column (Group 1) of the periodic table. These include lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr). Francium, being highly radioactive and short-lived, is less frequently discussed in practical applications.
These elements are characterized by several key properties:
1. Electronic Configuration and Valence Electrons:
The defining characteristic of alkali metals is their electronic configuration. They all possess one valence electron in their outermost shell. This single electron is relatively loosely held, making these elements highly reactive and prone to losing this electron to achieve a stable noble gas configuration. This tendency to readily lose an electron is what dictates their chemical behavior.
2. Metallic Properties:
Alkali metals exhibit classic metallic properties. They are soft, silvery-white solids (except for cesium, which has a slightly yellowish tint) with low densities and melting points. This softness is directly attributed to the weak metallic bonding resulting from the single valence electron. Their electrical and thermal conductivity is exceptionally high, further reinforcing their metallic nature.
3. Reactivity and Oxidation States:
The single valence electron makes alkali metals extremely reactive. They readily react with water, oxygen, and halogens, often with vigorous reactions. This reactivity increases as you go down the group, with cesium being the most reactive. Their typical oxidation state is +1, meaning they tend to lose one electron to form a +1 ion. This ease of oxidation is a key factor in their applications and also necessitates careful handling to prevent dangerous reactions.
4. Reaction with Water:
The reaction of alkali metals with water is perhaps their most striking characteristic and a common demonstration in chemistry classrooms. The reaction is exothermic, meaning it releases heat, and often produces a visible flame. The intensity of this reaction increases down the group. Lithium reacts relatively gently, while sodium reacts vigorously, potassium even more so, and rubidium and cesium react explosively. The general equation for the reaction is:
2M(s) + 2H₂O(l) → 2MOH(aq) + H₂(g)
where M represents the alkali metal.
5. Reaction with Halogens:
Alkali metals also readily react with halogens (Group 17 elements like chlorine, bromine, and iodine) to form ionic compounds known as alkali metal halides. These reactions are highly exothermic and often release significant amounts of energy. For example, the reaction between sodium and chlorine produces sodium chloride (table salt):
2Na(s) + Cl₂(g) → 2NaCl(s)
Applications of Alkali Metals and Their Compounds: A Wide Range of Uses
The unique properties of alkali metals and their compounds translate into a diverse range of applications across many industries:
1. Lithium: Batteries and Beyond
Lithium, the lightest alkali metal, plays a critical role in modern technology, primarily as a component in lithium-ion batteries. These batteries power everything from smartphones and laptops to electric vehicles and grid-scale energy storage systems. The high energy density and relatively long lifespan of lithium-ion batteries make them ideal for portable electronics and electric vehicles. Beyond batteries, lithium compounds are also used in ceramics, glass, and lubricating greases.
2. Sodium: Everyday Uses and Industrial Processes
Sodium is arguably the most well-known alkali metal due to its presence in table salt (sodium chloride). Sodium compounds are ubiquitous, playing a role in numerous industrial processes, including the production of sodium hydroxide (lye), a key ingredient in soap manufacturing and paper production. Sodium lamps, known for their distinctive yellow light, are frequently used in street lighting. Sodium also has applications in the chemical industry as a reducing agent.
3. Potassium: Agriculture and Pharmaceuticals
Potassium is essential for plant growth and is a crucial nutrient found in fertilizers. Potassium compounds are vital for regulating plant water balance and enzyme activity. In the human body, potassium plays a crucial role in maintaining proper nerve function and muscle contractions. Potassium salts are used in various pharmaceutical preparations.
4. Rubidium and Cesium: Specialized Applications
Rubidium and cesium, while less common, find applications in specialized areas. Cesium is used in atomic clocks due to its precise atomic transitions. Both rubidium and cesium are used in photoelectric cells and other specialized electronic devices.
The Reactivity Trend: Exploring the Factors that Influence Alkali Metal Behavior
The reactivity of alkali metals significantly increases as you move down the group from lithium to cesium. This trend is attributed to several factors:
-
Atomic Radius: As you descend Group 1, the atomic radius increases. This means the outermost electron is further from the nucleus and is less strongly attracted to it. This makes it easier to remove the valence electron, leading to increased reactivity.
-
Ionization Energy: Ionization energy is the energy required to remove an electron from an atom. It decreases down the group, reflecting the weaker hold the nucleus has on the outermost electron. Lower ionization energy directly correlates with increased reactivity.
-
Electronegativity: Electronegativity measures an atom's ability to attract electrons in a chemical bond. Electronegativity decreases down the group, meaning alkali metals become less likely to attract electrons and more likely to lose their own.
Safety Precautions and Handling of Alkali Metals: A Necessary Consideration
Due to their high reactivity, alkali metals require careful handling. Exposure to air and moisture can lead to spontaneous combustion or violent reactions. The following safety measures are essential:
-
Storage: Alkali metals should be stored under inert atmospheres (like argon) to prevent reaction with oxygen and moisture.
-
Handling: Use appropriate personal protective equipment (PPE), including gloves, eye protection, and lab coats.
-
Disposal: Dispose of alkali metals and their waste according to established safety protocols.
-
Water Reactions: Avoid contact with water, as the reactions can be highly exothermic and dangerous.
Alkali Metals in Biological Systems: Essential Roles and Implications
Alkali metals play critical roles in various biological systems.
-
Sodium and Potassium in Nerve Impulses: Sodium and potassium ions are crucial for the transmission of nerve impulses. The movement of these ions across cell membranes creates electrical signals that allow for communication within the nervous system.
-
Potassium in Plant Growth: Potassium is essential for proper plant growth and development. It is involved in enzyme activation, protein synthesis, and other critical metabolic processes.
-
Sodium in Fluid Balance: Sodium plays a critical role in regulating fluid balance in the body.
Conclusion: The Significance of Alkali Metals in Our World
The alkali metals, while highly reactive, are integral components of numerous technologies and biological processes. Their unique properties and reactivity make them indispensable in various fields, from energy storage and medicine to agriculture and industrial manufacturing. Understanding their characteristics and handling them safely is crucial for harnessing their potential while mitigating the risks associated with their high reactivity. Future research into alkali metals and their compounds will undoubtedly uncover even more applications and expand our understanding of their significance in the natural world and technological advancements.
Latest Posts
Latest Posts
-
What Is 7 20 As A Percent
Mar 28, 2025
-
What Element Is Shiny And Conducts Heat And Electricity
Mar 28, 2025
-
3x 2y 6 In Slope Intercept Form
Mar 28, 2025
-
How To Find The Mass Of A Planet
Mar 28, 2025
-
What Percent Of 120 Is 42
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Group 1a Elements Are Also Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
