Explain How Ionic Compounds Dissolve In Water
listenit
Mar 16, 2025 · 5 min read
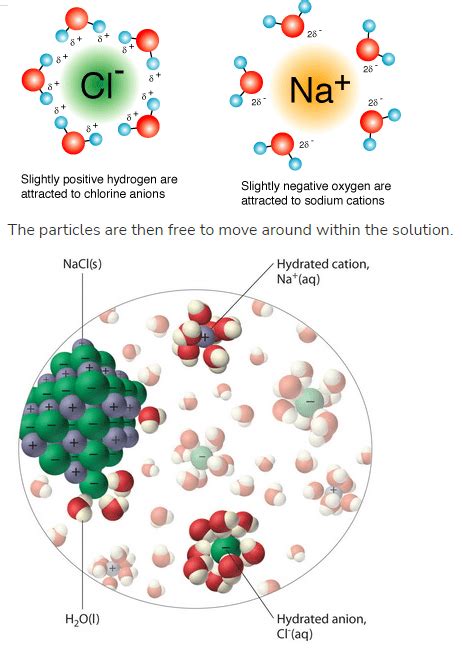
Table of Contents
How Ionic Compounds Dissolve in Water: A Deep Dive
Understanding how ionic compounds dissolve in water is crucial for grasping many fundamental chemical concepts. This process, known as dissolution, isn't simply about the solid disappearing; it's a complex interplay of electrostatic forces, molecular interactions, and thermodynamics. This comprehensive guide will explore the intricate details of ionic compound dissolution, from the microscopic perspective to its macroscopic implications.
The Nature of Ionic Compounds
Before delving into the dissolution process, it's essential to understand the structure and properties of ionic compounds. These compounds are formed through the electrostatic attraction between oppositely charged ions: cations (positively charged) and anions (negatively charged). This strong attraction creates a crystalline lattice structure, where ions are arranged in a highly ordered, three-dimensional array. The strength of this lattice depends on several factors, including the charges of the ions and the distance between them (Coulomb's Law). The stronger the electrostatic attraction, the higher the lattice energy, and the more difficult it is to break apart the crystal lattice. Examples of common ionic compounds include sodium chloride (NaCl, table salt), potassium iodide (KI), and calcium carbonate (CaCO₃).
Lattice Energy: The Key to Dissolution
Lattice energy is the energy required to completely separate one mole of a solid ionic compound into its gaseous ions. It's a measure of the strength of the ionic bonds within the crystal lattice. High lattice energy indicates strong ionic bonds, making it harder for the compound to dissolve. Conversely, low lattice energy implies weaker bonds, facilitating easier dissolution. Factors influencing lattice energy include:
- Charge of the ions: Higher charges lead to stronger electrostatic attraction and higher lattice energy.
- Size of the ions: Smaller ions have a shorter distance between their centers, resulting in stronger attraction and higher lattice energy.
The Role of Water: A Polar Solvent
Water (H₂O) is a remarkable solvent, particularly effective at dissolving ionic compounds. This ability stems from its polarity. The oxygen atom in a water molecule is more electronegative than the hydrogen atoms, creating a partial negative charge (δ-) on the oxygen and partial positive charges (δ+) on the hydrogens. This unequal distribution of charge creates a dipole moment, making water a polar molecule.
Water's Interaction with Ions: Hydration
The dissolution of an ionic compound in water is driven by the interaction between water molecules and the ions. This interaction is known as hydration. When an ionic compound is added to water, the polar water molecules surround the individual ions, effectively shielding them from each other. The partially negative oxygen atoms of water molecules are attracted to the cations, while the partially positive hydrogen atoms are attracted to the anions. This interaction weakens the electrostatic forces holding the crystal lattice together.
The Hydration Shell: A Protective Shield
Each ion becomes surrounded by a shell of water molecules, forming a hydration shell. The number of water molecules in the hydration shell depends on the size and charge of the ion. Smaller and more highly charged ions attract more water molecules, forming larger hydration shells. This hydration process is exothermic (releases energy) for many ionic compounds, contributing to the spontaneity of dissolution.
The Dissolution Process: Step-by-Step
The dissolution of an ionic compound in water can be visualized as a multi-step process:
- Dissociation: The ionic crystal lattice begins to break apart due to the interaction with water molecules. Water molecules penetrate the crystal lattice, weakening the electrostatic forces between the ions.
- Ion Separation: As the lattice weakens, individual ions become separated from the crystal structure. This step requires energy to overcome the lattice energy.
- Hydration: Once separated, the ions are surrounded by water molecules, forming hydration shells. This process releases energy (hydration enthalpy), counteracting the energy required for ion separation.
- Dispersion: The hydrated ions disperse throughout the solution, becoming evenly distributed.
The Enthalpy of Dissolution: A Thermodynamic Perspective
The overall enthalpy change (ΔH) during dissolution is the sum of the lattice energy and the hydration enthalpy. If the hydration enthalpy is greater than the lattice energy, the overall process is exothermic (ΔH < 0) and dissolution occurs spontaneously. However, if the lattice energy is greater than the hydration enthalpy, the process is endothermic (ΔH > 0), and dissolution may not occur spontaneously, or it may require an input of energy.
Factors Affecting the Solubility of Ionic Compounds
Several factors influence the solubility of ionic compounds in water:
- Lattice energy: As discussed, higher lattice energy reduces solubility.
- Hydration enthalpy: Higher hydration enthalpy increases solubility.
- Temperature: Solubility often increases with temperature, as the increased kinetic energy helps overcome the lattice energy.
- Common ion effect: The presence of a common ion in the solution reduces the solubility of the ionic compound.
- pH: The pH of the solution can affect the solubility of certain ionic compounds, particularly those containing weak acids or bases.
Applications and Importance
The understanding of how ionic compounds dissolve in water is crucial in various fields:
- Medicine: Dissolution is critical for drug delivery, as many medications are administered in aqueous solutions.
- Environmental science: Understanding the solubility of ionic compounds helps in assessing the environmental impact of pollutants and designing effective remediation strategies.
- Geochemistry: The dissolution and precipitation of ionic compounds play a significant role in geological processes, such as the formation of caves and mineral deposits.
- Industrial processes: Many industrial processes rely on the solubility of ionic compounds, such as the production of fertilizers and cleaning agents.
Conclusion
The dissolution of ionic compounds in water is a complex and fascinating process, driven by the interplay of electrostatic forces, molecular interactions, and thermodynamics. This process is fundamental to many natural and industrial phenomena and has far-reaching implications in various scientific and technological fields. Understanding the factors that influence solubility allows for better control and manipulation of these processes, leading to advancements in medicine, environmental science, and many other areas. The interplay between lattice energy and hydration enthalpy dictates whether an ionic compound will readily dissolve, providing a framework for predicting and understanding solubility behavior. Further exploration into the nuances of this process will continue to yield valuable insights into the behavior of matter at the molecular level.
Latest Posts
Latest Posts
-
What Is A Compound Represented By
Mar 16, 2025
-
What Is 23 Out Of 30
Mar 16, 2025
-
What Makes Up The Rungs Of Dna
Mar 16, 2025
-
How To Find The X Intercept Of A Parabola
Mar 16, 2025
-
How Many Inches Are In 1 4 Of A Yard
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about Explain How Ionic Compounds Dissolve In Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
