What Is A Compound Represented By
listenit
Mar 16, 2025 · 6 min read
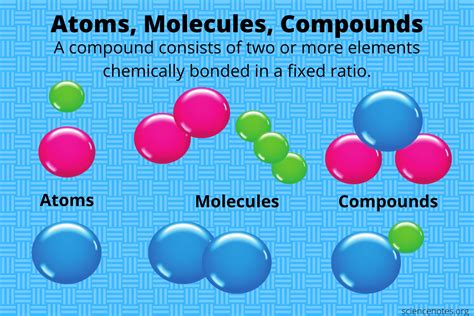
Table of Contents
What is a Compound Represented By? A Deep Dive into Chemical Formulas and Nomenclature
Understanding what a compound is represented by is fundamental to chemistry. It's not just about memorizing symbols; it's about grasping the underlying principles of chemical bonding, stoichiometry, and the language we use to communicate about the building blocks of matter. This article will delve deep into the various ways we represent chemical compounds, exploring their intricacies and significance.
Beyond the Basics: The Chemical Formula
At its core, a chemical compound is represented by a chemical formula. This formula is a concise way of representing the type and number of atoms present in a single molecule or formula unit of the compound. For example, H₂O represents water, indicating two hydrogen atoms and one oxygen atom. This seemingly simple representation encapsulates a wealth of information.
Types of Chemical Formulas: There are several types of chemical formulas, each offering a different level of detail:
-
Empirical Formula: This shows the simplest whole-number ratio of atoms in a compound. For example, the empirical formula for glucose (C₆H₁₂O₆) is CH₂O. This formula doesn't tell us the actual number of atoms in a molecule, only the ratio.
-
Molecular Formula: This shows the actual number of atoms of each element in a molecule. For glucose, the molecular formula is C₆H₁₂O₆. This provides complete information about the composition of a single molecule.
-
Structural Formula: This goes beyond simply listing atoms; it shows how the atoms are arranged and bonded within the molecule. This is particularly crucial for understanding the properties and reactivity of organic compounds. Structural formulas can be represented in various ways, including condensed formulas, skeletal formulas, and 3D models.
-
Condensed Structural Formula: This represents the connectivity of atoms in a molecule in a more compact form than a fully drawn structural formula. For example, ethanol might be written as CH₃CH₂OH.
-
Skeletal Formula (Line-angle Formula): This is a simplified representation of organic molecules where carbon atoms are implied at the intersections and ends of lines, and hydrogen atoms attached to carbons are omitted for brevity.
The Power of Nomenclature: Naming Compounds
Naming compounds (chemical nomenclature) is a systematic process that allows chemists worldwide to understand and communicate about specific substances. A well-defined name instantly provides information about the compound's composition and, often, its structure.
Inorganic Nomenclature: The naming of inorganic compounds follows specific rules depending on the type of compound:
-
Ionic Compounds: These compounds consist of cations (positively charged ions) and anions (negatively charged ions). The cation's name is written first, followed by the anion's name. For example, NaCl is sodium chloride. Roman numerals are used for transition metals to indicate their charge (e.g., FeCl₂ is iron(II) chloride, while FeCl₃ is iron(III) chloride).
-
Molecular Compounds (Covalent Compounds): These compounds are formed by the sharing of electrons between non-metal atoms. Prefixes (mono-, di-, tri-, tetra-, etc.) are used to indicate the number of atoms of each element. For example, CO₂ is carbon dioxide, and N₂O₄ is dinitrogen tetroxide.
-
Acids: Acids are compounds that release hydrogen ions (H⁺) in aqueous solutions. Their names often start with "hydro-" if they are derived from binary acids (containing only hydrogen and another non-metal), followed by the root name of the non-metal and "-ic acid." For example, HCl is hydrochloric acid. Oxoacids (containing oxygen) have more complex naming rules, often using prefixes like "hypo-" and "per-" and suffixes like "-ous acid" and "-ic acid."
Organic Nomenclature: The naming of organic compounds (compounds containing carbon) is more complex due to the vast number and variety of organic molecules. The International Union of Pure and Applied Chemistry (IUPAC) has developed a systematic nomenclature system to deal with this complexity. This system involves identifying the longest carbon chain (parent chain), identifying substituents (groups attached to the parent chain), numbering the carbon atoms, and arranging the name in a specific order.
Beyond the Formula: Understanding the Properties
The chemical formula, while crucial, only provides a partial picture of a compound. A compound's properties – physical and chemical – are determined by a multitude of factors, including:
-
Bonding Type: The type of chemical bond (ionic, covalent, metallic) significantly impacts the compound's properties. Ionic compounds tend to have high melting points and are often soluble in water, while covalent compounds exhibit a wider range of melting points and solubilities.
-
Molecular Structure: The arrangement of atoms in a molecule determines its shape, which in turn influences its properties. For example, the bent shape of a water molecule leads to its polar nature and high surface tension.
-
Intermolecular Forces: The forces of attraction between molecules (e.g., hydrogen bonding, dipole-dipole interactions, London dispersion forces) also contribute significantly to a compound's properties, affecting its melting point, boiling point, and solubility.
-
Isomerism: Compounds with the same molecular formula but different structural arrangements are called isomers. Isomers can have vastly different properties despite their identical chemical formulas. For example, glucose and fructose both have the molecular formula C₆H₁₂O₆, but they have different structures and properties.
Applications and Importance
The ability to represent and understand chemical compounds is essential in numerous fields:
-
Medicine: Understanding the chemical formulas and structures of drugs is crucial for their development, synthesis, and administration. Drug interactions and efficacy are directly related to the chemical properties of the compounds involved.
-
Materials Science: The properties of materials are directly linked to the chemical composition and structure of the compounds they are made of. This knowledge is vital in designing and synthesizing new materials with specific properties.
-
Environmental Science: Analyzing the chemical composition of pollutants and understanding their behavior in the environment requires a thorough understanding of chemical formulas and their properties.
-
Food Science: The chemical composition of food determines its nutritional value, taste, and texture. Understanding the chemical compounds present in food is vital for food safety and quality control.
Advanced Representations: Beyond the Basic Formula
While chemical formulas provide a fundamental representation, more sophisticated techniques are often employed to gain a deeper understanding of a compound:
-
Spectroscopy: Techniques like NMR (nuclear magnetic resonance), IR (infrared), and mass spectrometry provide detailed information about a compound's structure and composition, often complementing or verifying information obtained from chemical formulas.
-
Crystallography: X-ray crystallography allows scientists to determine the precise three-dimensional arrangement of atoms in a crystalline solid, providing invaluable insights into the compound's structure and properties.
-
Computational Chemistry: Computer simulations and modeling techniques allow scientists to predict and study the properties of molecules and compounds, particularly useful for complex systems where experimental investigation is challenging.
Conclusion: A Multifaceted Representation
The representation of a compound is not a single entity but a multifaceted approach encompassing chemical formulas, nomenclature systems, structural representations, and advanced analytical techniques. Understanding these various ways of representing compounds is fundamental to interpreting their properties, predicting their behavior, and applying this knowledge to diverse scientific fields. The seemingly simple chemical formula is a gateway to a world of complexity and potential, driving innovation and progress in countless areas. From the simplest molecules to the most complex biomolecules, a deep understanding of how we represent compounds is the cornerstone of chemical understanding.
Latest Posts
Latest Posts
-
Power Series Representation Of Ln 1 X
Mar 16, 2025
-
How Many Nm In One M
Mar 16, 2025
-
3 2 1 4s 5 3
Mar 16, 2025
-
Cuanto Es 54 Centimetros En Pulgadas
Mar 16, 2025
-
Where Is Most Of Earths Freshwater Located
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about What Is A Compound Represented By . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
