Equation For The Combustion Of Octane
listenit
Mar 21, 2025 · 5 min read
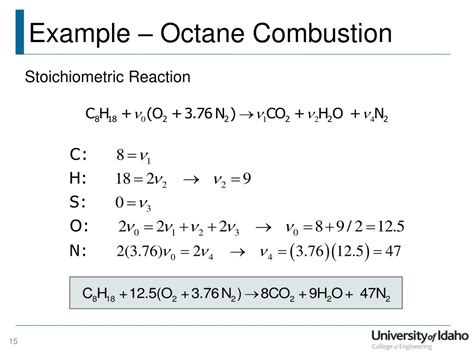
Table of Contents
The Equation for the Combustion of Octane: A Deep Dive
The combustion of octane, a key component of gasoline, is a fundamental chemical process crucial to understanding internal combustion engines and energy production. While the simple equation provides a basic understanding, a deeper dive reveals complexities and nuances that significantly impact efficiency, emissions, and environmental concerns. This article explores the combustion equation, its variations, influencing factors, and the broader implications of this vital reaction.
The Simplified Combustion Equation of Octane
The most common and simplified representation of octane combustion is:
2C₈H₁₈ + 25O₂ → 16CO₂ + 18H₂O
This equation illustrates the stoichiometric combustion of octane (C₈H₁₈) with oxygen (O₂), resulting in the production of carbon dioxide (CO₂) and water (H₂O). "Stoichiometric" means that the reactants are present in the exact proportions needed for complete combustion – meaning all the octane is consumed, and no excess oxygen or fuel remains.
Understanding the Equation
Let's break down what this equation tells us:
- 2C₈H₁₈: Two molecules of octane are involved in the reaction. This is because balancing the equation requires an even number of carbon and hydrogen atoms.
- 25O₂: Twenty-five molecules of oxygen are necessary to completely oxidize the two octane molecules. This highlights the significant oxygen requirement for complete combustion.
- 16CO₂: Sixteen molecules of carbon dioxide are produced as a byproduct of the reaction. This is the primary greenhouse gas resulting from the combustion of octane.
- 18H₂O: Eighteen molecules of water are also produced. While not a greenhouse gas, the production of water contributes to the overall energy balance and potentially affects humidity levels in certain applications.
The Reality: Incomplete Combustion and Other Products
The simplified equation, while useful for basic understanding, doesn't fully capture the complexity of real-world octane combustion. In reality, combustion rarely achieves perfect stoichiometry. Several factors can lead to incomplete combustion:
- Insufficient Oxygen: A lack of sufficient oxygen results in the production of carbon monoxide (CO), a highly toxic gas. The equation for incomplete combustion can vary greatly depending on the oxygen-to-fuel ratio. A possible example is:
2C₈H₁₈ + 17O₂ → 16CO + 18H₂O
This illustrates the formation of CO when oxygen is limited.
-
Low Temperatures: Lower temperatures prevent the complete oxidation of fuel molecules. This also leads to incomplete combustion and increased CO production, along with the formation of unburnt hydrocarbons (UHCs).
-
Rapid Combustion: In engines, combustion happens extremely rapidly. This fast process can lead to local variations in oxygen concentration and temperature, causing incomplete combustion in some regions.
Other Combustion Products
Besides CO and UHCs, incomplete combustion can also produce other harmful byproducts, including:
- Nitrogen Oxides (NOx): High temperatures within the combustion chamber cause nitrogen from the air to react with oxygen, forming NOx. These are significant air pollutants contributing to acid rain and smog.
- Particulate Matter (PM): Incomplete combustion also generates soot and other particulate matter, which have adverse health effects.
- Soot (Elemental Carbon): This is a byproduct of the incomplete combustion process, particularly at fuel-rich conditions.
The presence and proportions of these byproducts depend significantly on various factors including the air-fuel ratio, combustion temperature, pressure, and the engine design.
Factors Influencing Octane Combustion
Several factors can significantly influence the combustion process, impacting efficiency, emissions, and performance:
-
Air-Fuel Ratio (AFR): The ratio of air (oxygen) to fuel is critical. A stoichiometric AFR is approximately 14.7:1 for octane (meaning 14.7 kg of air for every 1 kg of octane). Leaner (more air) or richer (more fuel) mixtures significantly affect combustion characteristics.
-
Temperature: The temperature within the combustion chamber directly affects the reaction rate and the completeness of combustion. Higher temperatures generally promote more complete combustion.
-
Pressure: Increased pressure increases the reaction rate and enhances complete combustion, leading to improved efficiency.
-
Engine Design: Engine features such as spark plug placement, compression ratio, and the overall design of the combustion chamber impact the efficiency and completeness of combustion.
-
Fuel Additives: Additives can influence the combustion process by altering the fuel's properties. For instance, octane boosters can improve combustion efficiency and reduce knocking.
Advanced Combustion Models and Analysis
The simplified equation provides a fundamental framework, but accurate modeling of real-world octane combustion requires significantly more complex approaches. These advanced models often employ computational fluid dynamics (CFD) and detailed chemical kinetics to predict the behavior of the combustion process. These models account for:
-
Turbulence: Turbulence within the combustion chamber mixes the fuel and air, significantly influencing combustion characteristics.
-
Heat Transfer: Heat transfer within the combustion chamber influences temperatures and reaction rates.
-
Chemical Kinetics: Detailed chemical kinetics models account for the numerous intermediate reactions involved in the combustion process, providing a more accurate prediction of product formation.
Environmental Implications
The combustion of octane, and the resultant emissions, have profound environmental implications. The production of carbon dioxide contributes to climate change, while other pollutants such as NOx and PM harm air quality and human health. The development and implementation of emission control technologies, such as catalytic converters, are crucial to mitigate the negative environmental impact of octane combustion. Research into alternative fuels and engine technologies continues to explore ways to reduce reliance on octane and minimize its environmental footprint.
Conclusion
The simple equation for the combustion of octane, 2C₈H₁₈ + 25O₂ → 16CO₂ + 18H₂O, serves as a starting point for understanding this vital chemical process. However, a comprehensive understanding necessitates acknowledging the complexities of incomplete combustion, the influence of various factors on the reaction, and the significant environmental implications. Advanced combustion modeling and ongoing research are essential to improve combustion efficiency, minimize harmful emissions, and develop more sustainable transportation solutions. The future of combustion technology lies in striving for cleaner, more efficient, and environmentally friendly methods of energy conversion, building upon the foundational knowledge provided by this seemingly simple equation. Continuous innovation in engine design, fuel formulations, and emissions control systems will be crucial in mitigating the negative consequences of octane combustion and advancing towards a more sustainable future.
Latest Posts
Latest Posts
-
What Is 10 3 As A Decimal
Mar 22, 2025
-
In What Unit Is Frequency Measured
Mar 22, 2025
-
How Many Ouces In A Pint
Mar 22, 2025
-
Rotated 180 Degrees About The Origin
Mar 22, 2025
-
Which Change Of State Involves A Release Of Energy
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Equation For The Combustion Of Octane . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
