Enzymes Are Composed Of What Organic Molecule
listenit
Mar 25, 2025 · 5 min read
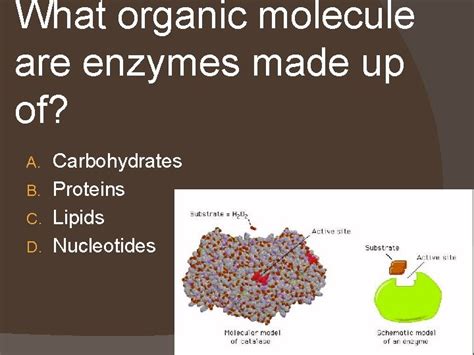
Table of Contents
Enzymes: Composed Entirely of Proteins (Mostly!)
Enzymes are the workhorses of life, the biological catalysts that drive countless chemical reactions within living organisms. Understanding their composition is crucial to grasping their function and the intricate mechanisms that underpin biological processes. While the simple answer is that enzymes are composed primarily of proteins, the reality is a bit more nuanced. This article will delve into the detailed composition of enzymes, exploring the roles of proteins, and occasionally other molecules, in their structure and function.
The Predominant Role of Proteins in Enzyme Structure
The vast majority of enzymes are proteins, meaning they are composed of long chains of amino acids linked together by peptide bonds. These chains, known as polypeptide chains, fold into intricate three-dimensional structures, dictated by the sequence of amino acids. This precise three-dimensional structure is absolutely crucial to enzyme function. The active site, the specific region of the enzyme where the substrate (the molecule the enzyme acts upon) binds, is a product of this intricate folding.
Amino Acid Composition and its Significance
The amino acid sequence of a protein determines its primary structure. This sequence then dictates how the protein folds into its secondary, tertiary, and quaternary structures. The 20 standard amino acids, each with unique chemical properties (polarity, charge, size, etc.), contribute to the overall properties of the enzyme. Some amino acids contribute to the active site directly, while others contribute to the overall stability and three-dimensional shape of the enzyme.
- Hydrophobic interactions: Nonpolar amino acids tend to cluster together in the enzyme's interior, away from the aqueous environment, contributing to the protein's stability.
- Hydrogen bonds: These weak bonds form between polar amino acids, helping to stabilize secondary structures like alpha-helices and beta-sheets.
- Disulfide bonds: Covalent bonds between cysteine residues create strong cross-links, reinforcing the tertiary structure of the enzyme.
- Ionic interactions: Attractive forces between oppositely charged amino acid side chains further stabilize the enzyme's three-dimensional structure.
Enzyme Classification Based on Protein Structure
Enzymes are often classified based on their protein structure:
- Simple Enzymes: These enzymes consist solely of polypeptide chains.
- Conjugated Enzymes (Holoenzymes): These enzymes consist of a protein component (apoenzyme) and a non-protein component (cofactor). The cofactor can be a metal ion (e.g., zinc, iron, magnesium) or a smaller organic molecule called a coenzyme. Coenzymes often derive from vitamins.
The Role of Cofactors in Enzyme Function
While proteins form the backbone of most enzymes, certain enzymes require additional components, known as cofactors, to function effectively. These cofactors can significantly enhance enzymatic activity or even be essential for catalysis.
Metal Ions as Cofactors
Metal ions often act as cofactors by:
- Stabilizing the enzyme's structure: They can interact with amino acid side chains, enhancing the stability of the protein's three-dimensional structure.
- Participating directly in catalysis: They may participate in redox reactions (electron transfer), or act as Lewis acids, accepting electron pairs from substrates.
- Binding substrates: Metal ions can bind to substrates, increasing their affinity for the active site.
Examples of enzymes requiring metal ions include:
- Carbonic anhydrase: Requires zinc for catalysis.
- Cytochrome c oxidase: Requires iron and copper for electron transport.
- DNA polymerase: Requires magnesium for proper function.
Coenzymes as Cofactors
Coenzymes are organic molecules that often act as transient carriers of electrons, atoms, or functional groups during the catalytic process. They are typically derived from vitamins.
- NAD+ and FAD: These coenzymes are involved in redox reactions, carrying electrons between different enzymes in metabolic pathways.
- Coenzyme A: This coenzyme plays a crucial role in metabolism, particularly in the transfer of acyl groups.
- Thiamine pyrophosphate (TPP): A coenzyme derived from thiamine (vitamin B1), involved in carbohydrate metabolism.
The coenzyme temporarily binds to the enzyme, participates in the reaction, and then dissociates, leaving the enzyme free to catalyze another reaction.
Beyond Proteins: Exceptions to the Rule
While the vast majority of enzymes are protein-based, a few exceptions exist. Some RNA molecules, called ribozymes, possess catalytic activity. These ribozymes are typically involved in RNA processing, such as splicing and self-cleavage.
Ribozymes: Catalytic RNA Molecules
Ribozymes challenge the notion that only proteins can catalyze biochemical reactions. Their discovery demonstrated the catalytic potential of RNA, providing insights into the early stages of life when RNA may have played a more central role in both genetic information storage and catalysis. Although less common than protein enzymes, ribozymes illustrate the diversity of biological catalysts.
Enzyme Structure and Function: An Inseparable Duo
The detailed composition of enzymes, primarily proteins with occasional cofactors, directly influences their function. The precise three-dimensional structure created by the amino acid sequence and interactions with cofactors creates the active site, a region perfectly tailored to bind a specific substrate. The active site's unique architecture allows for specific substrate recognition and the efficient catalysis of a particular chemical reaction. Any changes to the enzyme's structure, whether through mutations in the amino acid sequence or alterations in the environment (e.g., temperature, pH), can significantly impact its catalytic activity.
The Importance of Enzyme Specificity
Enzyme specificity is a defining characteristic, resulting from the precise interaction between the enzyme's active site and its substrate. This specificity ensures that enzymes catalyze only the intended reactions, preventing unwanted side reactions and maintaining the intricate balance of metabolic pathways within the cell.
Enzyme Kinetics and Catalytic Mechanisms
The study of enzyme kinetics explores the rate of enzyme-catalyzed reactions, providing insights into the mechanisms by which enzymes accelerate biochemical reactions. This involves understanding how substrate concentration, enzyme concentration, temperature, and pH affect reaction rates. Different enzyme catalytic mechanisms exist, such as acid-base catalysis, covalent catalysis, and metal ion catalysis.
Conclusion: A Complex but Crucial Biological Machinery
In conclusion, while the primary component of most enzymes is protein, understanding their composition requires a deeper dive into the roles of amino acids, cofactors (metal ions and coenzymes), and the occasional RNA-based ribozyme. The precise arrangement of these components determines the enzyme's three-dimensional structure, which, in turn, dictates its substrate specificity and catalytic efficiency. This intricate interplay of structure and function makes enzymes indispensable for life, driving the countless chemical reactions necessary for maintaining cellular homeostasis and supporting the complex processes of living organisms. Further research into enzyme structure and function will undoubtedly reveal even more about the remarkable precision and efficiency of this biological machinery.
Latest Posts
Latest Posts
-
Is The Elbow Proximal To The Shoulder
Mar 28, 2025
-
Is Evaporation A Chemical Or Physical Change
Mar 28, 2025
-
Which Is More Dense Oceanic Or Continental Crust
Mar 28, 2025
-
Classify These Orbital Descriptions By Type
Mar 28, 2025
-
What Is Square Root Of 625
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Enzymes Are Composed Of What Organic Molecule . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
