Does Ethylene Glycol Have Dipole Dipole Forces
listenit
Mar 30, 2025 · 6 min read
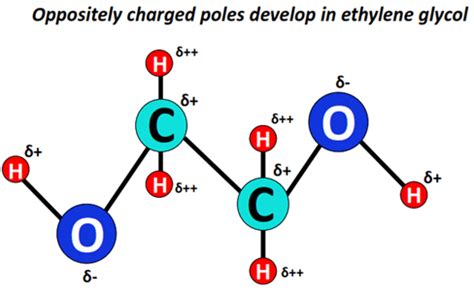
Table of Contents
Does Ethylene Glycol Have Dipole-Dipole Forces? A Deep Dive into Molecular Interactions
Ethylene glycol, a common ingredient in antifreeze and other industrial applications, possesses a fascinating molecular structure that leads to a rich array of intermolecular forces. Understanding these forces is crucial to predicting its physical properties, such as its high boiling point and solubility in water. One key question often arises: Does ethylene glycol have dipole-dipole forces? The answer, as we'll explore in detail, is a resounding yes, but the story is far more nuanced than a simple yes or no.
Understanding Dipole-Dipole Forces
Before delving into the specifics of ethylene glycol, let's establish a solid foundation in dipole-dipole forces. These forces are a type of intermolecular force that arises between polar molecules. A polar molecule is one that possesses a permanent dipole moment, meaning there's an uneven distribution of electron density across the molecule. This uneven distribution results from a difference in electronegativity between the atoms within the molecule. Electronegativity refers to the ability of an atom to attract electrons towards itself in a chemical bond.
A classic example of a polar molecule is water (H₂O). The oxygen atom is significantly more electronegative than the hydrogen atoms, leading to a partial negative charge (δ-) on the oxygen and partial positive charges (δ+) on the hydrogens. This creates a dipole, a separation of positive and negative charges within the molecule. Dipole-dipole forces are the attractive forces between the positive end of one polar molecule and the negative end of another. These forces are relatively strong compared to other intermolecular forces like London dispersion forces, but weaker than hydrogen bonds.
The Molecular Structure of Ethylene Glycol
Ethylene glycol (C₂H₆O₂) has the chemical formula HOCH₂CH₂OH. Its structure is characterized by two hydroxyl (-OH) groups attached to adjacent carbon atoms. This arrangement is crucial in determining its intermolecular forces. Let's break down the key features:
-
Hydroxyl Groups (-OH): The hydroxyl groups are highly polar due to the significant difference in electronegativity between oxygen and hydrogen. The oxygen atom attracts electrons more strongly, creating a partial negative charge on the oxygen and a partial positive charge on the hydrogen.
-
Carbon-Oxygen Bonds: The carbon-oxygen bonds (C-O) are also polar, although less so than the O-H bonds. Oxygen's higher electronegativity leads to a partial negative charge on the oxygen and a partial positive charge on the carbon.
-
Overall Molecular Polarity: The combined effect of the polar O-H and C-O bonds results in a significant overall dipole moment for the ethylene glycol molecule. The molecule is not symmetrical; the two hydroxyl groups are on opposite sides of the molecule, contributing to this net dipole moment.
The Presence of Dipole-Dipole Forces in Ethylene Glycol
Given the significant polarity of ethylene glycol stemming from its hydroxyl groups and C-O bonds, it's clear that dipole-dipole forces are present. The partial positive charges on the hydrogens of the hydroxyl groups are attracted to the partial negative charges on the oxygens of neighboring ethylene glycol molecules. Similarly, the partial positive charges on the carbons are attracted to the partial negative charges on the oxygens. These dipole-dipole interactions contribute substantially to the physical properties of ethylene glycol.
Beyond Dipole-Dipole Forces: Hydrogen Bonding
While dipole-dipole forces are present, it's crucial to acknowledge the even stronger intermolecular forces at play in ethylene glycol: hydrogen bonding. Hydrogen bonding is a special type of dipole-dipole interaction that occurs when a hydrogen atom is bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) and is attracted to another electronegative atom in a nearby molecule.
In ethylene glycol, the hydrogen atoms of the hydroxyl groups can form hydrogen bonds with the oxygen atoms of other ethylene glycol molecules. This results in a strong network of intermolecular interactions that significantly affect its physical properties. The strength of hydrogen bonding in ethylene glycol contributes significantly to its relatively high boiling point (197.3 °C) compared to molecules of similar size that lack hydrogen bonding. This strong intermolecular attraction requires more energy to overcome, hence the higher boiling point.
The Role of London Dispersion Forces
Even though dipole-dipole forces and hydrogen bonding are dominant, we can't ignore the contribution of London dispersion forces. These forces are the weakest type of intermolecular force and arise from temporary, instantaneous fluctuations in electron distribution around atoms and molecules. Even nonpolar molecules experience London dispersion forces.
In ethylene glycol, while less significant compared to hydrogen bonding and dipole-dipole forces, London dispersion forces still play a role in the overall intermolecular interactions. They contribute to the overall cohesive energy of the liquid.
Ethylene Glycol's Solubility in Water: A Consequence of Intermolecular Forces
The significant solubility of ethylene glycol in water is a direct consequence of the strong intermolecular forces present. Water itself is a highly polar molecule with strong hydrogen bonding capabilities. Ethylene glycol, with its own hydroxyl groups and capacity for hydrogen bonding, can readily interact with water molecules through hydrogen bonding. The hydrogen bonds formed between ethylene glycol and water molecules overcome the intermolecular forces within the individual substances, allowing for easy mixing. This strong interaction explains the complete miscibility of ethylene glycol and water.
Comparing Ethylene Glycol's Intermolecular Forces with Other Molecules
Let's compare ethylene glycol's intermolecular forces to those of similar molecules:
-
Ethanol (C₂H₅OH): Ethanol has one hydroxyl group, resulting in weaker hydrogen bonding and dipole-dipole forces compared to ethylene glycol. Its boiling point is consequently lower.
-
Diethyl ether (C₂H₅OC₂H₅): Diethyl ether lacks hydroxyl groups and primarily exhibits dipole-dipole forces and London dispersion forces. Its boiling point is considerably lower than ethylene glycol's.
-
Methane (CH₄): Methane is a nonpolar molecule, and only London dispersion forces are present. Its boiling point is significantly lower.
This comparison highlights the significant role of the two hydroxyl groups in ethylene glycol's strong intermolecular forces, leading to its higher boiling point and greater solubility in water.
Applications Leveraging Ethylene Glycol's Properties
The unique combination of intermolecular forces in ethylene glycol underpins its many applications:
-
Antifreeze: Its high boiling point and low freezing point make it ideal for preventing the freezing and boiling of water in car radiators.
-
Coolant: It's used in industrial cooling systems due to its effective heat transfer properties.
-
Solvent: Its polarity makes it a good solvent for many polar compounds.
-
Plastics Production: It's a key component in the production of certain types of plastics.
These applications are all directly related to the strong intermolecular forces that govern its physical properties.
Conclusion: A Comprehensive Picture
In conclusion, ethylene glycol undoubtedly exhibits dipole-dipole forces due to its polar hydroxyl groups and C-O bonds, contributing to its overall polarity. However, the story doesn't end there. The dominant intermolecular forces are hydrogen bonds, which are significantly stronger and account for its high boiling point and solubility in water. While London dispersion forces are present, their impact is comparatively less significant. Understanding the interplay of these intermolecular forces provides a complete picture of ethylene glycol's properties and its diverse applications. This detailed analysis underscores the importance of considering all types of intermolecular forces when predicting and explaining the behavior of molecules.
Latest Posts
Latest Posts
-
How To Go From Grams To Molecules
Apr 01, 2025
-
3 As A Percentage Of 8
Apr 01, 2025
-
What Is 20 Off Of 600
Apr 01, 2025
-
Why Is Dna Replication Described As Semi Conservative
Apr 01, 2025
-
Atoms That Gain Or Lose Electrons Are Called
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Does Ethylene Glycol Have Dipole Dipole Forces . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
