Does Electronegativity Increase Down A Group
listenit
Mar 22, 2025 · 4 min read
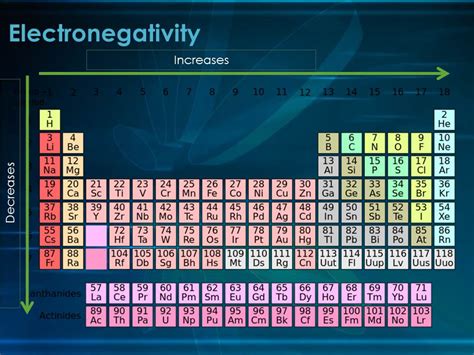
Table of Contents
Does Electronegativity Increase Down a Group? Understanding Periodic Trends
Electronegativity, a fundamental concept in chemistry, describes an atom's ability to attract electrons towards itself within a chemical bond. Understanding how electronegativity changes across the periodic table is crucial for predicting the nature of chemical bonds and the properties of molecules. A common question that arises is: Does electronegativity increase down a group? The short answer is no, it generally decreases down a group. Let's delve deeper into this trend, exploring the reasons behind it and examining its implications.
Understanding Electronegativity
Before exploring the group trend, it's vital to grasp the concept of electronegativity itself. Electronegativity is a relative property; it's a comparison of how strongly an atom attracts electrons compared to other atoms. Several scales exist to quantify electronegativity, the most commonly used being the Pauling scale. On this scale, fluorine (F), the most electronegative element, is assigned a value of 4.0.
Several factors influence an atom's electronegativity:
- Nuclear Charge: A higher positive charge on the nucleus exerts a stronger pull on electrons.
- Atomic Radius: A smaller atomic radius means electrons are closer to the nucleus, experiencing a stronger attractive force.
- Shielding Effect: Inner electrons shield outer electrons from the full positive charge of the nucleus, reducing the effective nuclear charge experienced by the valence electrons.
Why Electronegativity Decreases Down a Group
The decrease in electronegativity down a group is primarily due to the increase in atomic radius. As you move down a group, the number of electron shells increases. This means that the valence electrons are further away from the nucleus. The increased distance significantly weakens the electrostatic attraction between the nucleus and the valence electrons.
While the nuclear charge also increases down a group (adding more protons), the effect of the increased shielding by the additional inner electrons outweighs the increased nuclear charge. The added electrons in the inner shells effectively shield the valence electrons from the increased nuclear pull. This shielding effect, coupled with the greater distance, results in a weaker attraction for electrons and hence, a lower electronegativity.
Visualizing the Trend: Examples
Let's illustrate the decrease in electronegativity down a group with specific examples:
Group 1 (Alkali Metals):
- Lithium (Li): Electronegativity ≈ 0.98
- Sodium (Na): Electronegativity ≈ 0.93
- Potassium (K): Electronegativity ≈ 0.82
- Rubidium (Rb): Electronegativity ≈ 0.82
- Cesium (Cs): Electronegativity ≈ 0.79
Notice the clear downward trend in electronegativity values. As we move down Group 1, the atomic radius increases significantly, leading to a decrease in electronegativity.
Group 17 (Halogens):
- Fluorine (F): Electronegativity ≈ 3.98
- Chlorine (Cl): Electronegativity ≈ 3.16
- Bromine (Br): Electronegativity ≈ 2.96
- Iodine (I): Electronegativity ≈ 2.66
- Astatine (At): Electronegativity ≈ 2.2
Again, we observe a consistent decrease in electronegativity down the group. Despite the increasing nuclear charge, the shielding effect and increased atomic radius dominate, resulting in a weaker attraction for bonding electrons.
Implications of Decreasing Electronegativity Down a Group
The decrease in electronegativity down a group has several important chemical implications:
-
Reactivity: Alkali metals become more reactive down the group. Their low electronegativity means they readily lose their valence electron, forming stable +1 cations. This explains why cesium is more reactive than lithium.
-
Bond Polarity: The difference in electronegativity between two atoms determines the polarity of a bond. When a bond is formed between atoms with significantly different electronegativities, the bond is polar (e.g., H-Cl). As electronegativity decreases down a group, the polarity of bonds involving elements from that group will decrease.
-
Oxidizing Power: Halogens are strong oxidizing agents, readily gaining an electron to achieve a stable noble gas configuration. However, their oxidizing power decreases down the group. Fluorine is the strongest oxidizing agent, while astatine is the weakest. This is directly linked to their decreasing electronegativity.
-
Acid Strength: The acidity of binary acids (e.g., HCl, HBr, HI) increases down the group. This is because the larger halide ions are more stable, making the release of the proton (H⁺) easier. This trend is linked to the decreasing electronegativity; less electronegative halogens stabilize the negative charge on the conjugate base more effectively.
Exceptions and Considerations
While the general trend is a decrease in electronegativity down a group, there can be minor deviations. These deviations are often subtle and can be attributed to variations in effective nuclear charge due to subtle differences in electron configurations or relativistic effects in heavier elements. However, the overall trend remains consistent.
Electronegativity Across a Period
It's important to contrast the trend down a group with the trend across a period (left to right). Electronegativity generally increases across a period. This is because the atomic radius decreases across a period, and the effective nuclear charge increases, leading to a stronger attraction for electrons.
Conclusion
The question, "Does electronegativity increase down a group?" is answered with a definitive no. Electronegativity generally decreases down a group due to the increasing atomic radius and the shielding effect of inner electrons. This trend has significant implications for various chemical properties, influencing reactivity, bond polarity, oxidizing power, and acid strength. Understanding this fundamental periodic trend is crucial for predicting and interpreting the behavior of elements and their compounds. The interplay between electronegativity and other periodic trends provides a rich foundation for understanding the complex world of chemistry.
Latest Posts
Latest Posts
-
How Many Centimeters Is 4 Meters
Mar 23, 2025
-
How Many Valence Electrons Does Sulfer Have
Mar 23, 2025
-
What Is 70 Percent Of 45
Mar 23, 2025
-
Is Force A Vector Or Scalar
Mar 23, 2025
-
How Many Pounds In 40 Ounces
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Does Electronegativity Increase Down A Group . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
