Do Ionic Compounds Have A High Melting Point
listenit
Mar 18, 2025 · 5 min read
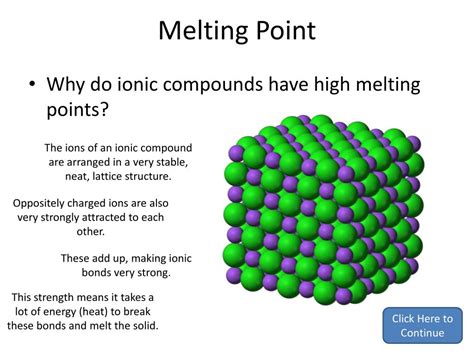
Table of Contents
Do Ionic Compounds Have a High Melting Point? Exploring the Strong Bonds Behind High Melting Points
Ionic compounds are renowned for their high melting points, a characteristic stemming from the strong electrostatic forces holding their constituent ions together. This article delves deep into the reasons behind this property, exploring the nature of ionic bonding, the factors influencing melting point variations, and exceptions to the rule. We'll also touch upon the practical implications of this characteristic in various applications.
Understanding Ionic Bonding: The Foundation of High Melting Points
The exceptionally high melting points of ionic compounds are directly linked to the nature of their bonding. Unlike covalent compounds where atoms share electrons, ionic compounds are formed through the electrostatic attraction between positively charged ions (cations) and negatively charged ions (anions). This attraction, known as an ionic bond, is significantly stronger than the intermolecular forces found in covalent compounds.
The Role of Electrostatic Forces: A Powerful Bond
The strength of the ionic bond depends on several crucial factors:
-
Charge Magnitude: Higher charges on the ions lead to a stronger electrostatic attraction. For example, the bond in magnesium oxide (MgO), with Mg²⁺ and O²⁻ ions, is stronger than the bond in sodium chloride (NaCl), with Na⁺ and Cl⁻ ions. This directly translates to a higher melting point for MgO.
-
Ionic Radius: Smaller ions lead to stronger bonds. When ions are smaller, the distance between their nuclei is reduced, resulting in a more potent electrostatic attraction. Conversely, larger ions experience weaker electrostatic forces, leading to lower melting points.
This interplay between charge magnitude and ionic radius dictates the overall strength of the ionic bond and consequently, the melting point.
Factors Influencing Melting Point Variations in Ionic Compounds
While the fundamental principle of strong ionic bonds explains the generally high melting points, variations exist among different ionic compounds. Several factors contribute to these differences:
1. Lattice Energy: The Energy of Attraction
Lattice energy is the energy required to completely separate one mole of a solid ionic compound into its gaseous ions. A higher lattice energy indicates stronger ionic bonds and, therefore, a higher melting point. Compounds with high lattice energies, like those with small, highly charged ions, will exhibit high melting points.
2. Coordination Number and Crystal Structure: Order and Arrangement
The arrangement of ions in a crystal lattice, also known as its crystal structure, impacts the overall strength of the ionic interactions. The coordination number, representing the number of ions surrounding a central ion, contributes to the overall stability of the lattice. Different crystal structures have varying coordination numbers, leading to differences in melting points, even for compounds with similar charge magnitudes and ionic radii.
3. Polarizability of Ions: Distorting the Charges
While less significant than charge and size, the polarizability of ions can subtly influence melting points. Polarizable ions can distort the electron cloud, creating temporary dipoles and slightly weakening the electrostatic attraction. This effect is more pronounced with larger ions, as their electron clouds are more easily distorted.
Exceptions and Deviations: When Ionic Compounds Melt at Lower Temperatures
Although ionic compounds are generally characterized by high melting points, exceptions exist. Several factors can lead to lower melting points than expected:
1. Weak Ionic Bonds: Small Charge, Large Size
Compounds formed with ions of low charge and large size will have relatively weak ionic bonds. This results in lower lattice energies and correspondingly lower melting points. Consider compounds with large monovalent ions like cesium iodide (CsI), which has a lower melting point compared to compounds with smaller, highly charged ions.
2. Covalent Character: Sharing the Electrons
Some ionic compounds exhibit a degree of covalent character. This arises when there's significant overlap between the electron clouds of the cation and anion. This sharing of electrons reduces the purely ionic nature of the bond, weakening the electrostatic attraction and lowering the melting point. This is more common with compounds involving highly charged cations with relatively small size and anions with high polarizability.
3. Presence of Water Molecules: Hydration Effects
Hydrated ionic compounds, containing water molecules within their crystal structure, often have lower melting points than their anhydrous counterparts. The water molecules interfere with the strong ionic interactions, weakening the lattice and reducing the melting point.
Practical Implications of High Melting Points in Ionic Compounds
The high melting points of ionic compounds have significant implications in various applications:
-
High-Temperature Applications: Materials with high melting points are crucial in high-temperature environments. Ionic compounds find use in refractory materials, components designed to withstand extreme heat in furnaces, kilns, and other high-temperature industrial processes.
-
Electrolytes: Many ionic compounds readily dissociate into ions when dissolved or melted, making them excellent electrolytes. This property is critical in batteries, fuel cells, and other electrochemical devices.
-
Ceramics and Glasses: Ionic compounds form the basis of many ceramic and glass materials. Their high melting points contribute to the durability and thermal stability of these materials.
-
Mineral Formation and Geology: The stability and high melting points of many ionic compounds are fundamental to geological processes. They are essential components of rocks and minerals, determining their properties and behavior under various conditions.
Conclusion: The Enduring Significance of Strong Ionic Bonds
The high melting points of ionic compounds are a direct consequence of the strong electrostatic forces between their constituent ions. This property, determined by factors like charge magnitude, ionic radius, lattice energy, and crystal structure, plays a significant role in their diverse applications and their importance in various fields, from materials science to geology. While exceptions exist, the overarching principle remains: strong ionic bonds translate to high melting points, highlighting the fundamental connection between bonding and macroscopic properties in ionic compounds. Understanding these relationships is essential for designing and utilizing materials with specific thermal and electrochemical properties.
Latest Posts
Latest Posts
-
How Many Atoms Are In A Door Per Cubic Meter
Mar 19, 2025
-
1 In 7 As A Percentage
Mar 19, 2025
-
Practice Problems For Completing The Square
Mar 19, 2025
-
Is Ice Melts A Chemical Change
Mar 19, 2025
-
How Many Pounds Is A Quarter Ton
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Do Ionic Compounds Have A High Melting Point . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
