Do Ionic Bonds Have High Solubility
listenit
Mar 17, 2025 · 5 min read
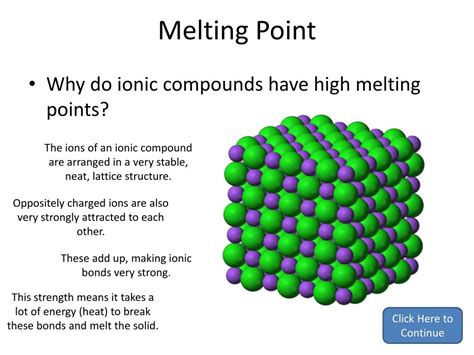
Table of Contents
Do Ionic Bonds Have High Solubility? Exploring the Factors that Influence Solubility
Ionic compounds, characterized by the strong electrostatic attraction between oppositely charged ions, exhibit a fascinating range of solubility behaviors. While many ionic compounds readily dissolve in polar solvents like water, others show limited or no solubility. This article delves deep into the intricate relationship between ionic bonding and solubility, examining the key factors that govern the dissolution process and exploring the exceptions to the general rule.
Understanding Ionic Bonds and Their Properties
Before we delve into solubility, let's solidify our understanding of ionic bonds. Ionic bonds form when a highly electronegative atom (like oxygen, chlorine, or fluorine) essentially steals one or more electrons from a less electronegative atom (like sodium, magnesium, or potassium). This electron transfer creates ions: positively charged cations and negatively charged anions. The powerful electrostatic forces of attraction between these oppositely charged ions constitute the ionic bond, resulting in a crystalline structure where ions are arranged in a highly ordered, three-dimensional lattice.
This lattice structure is crucial in determining an ionic compound's properties, including its solubility. The strength of the ionic bonds within the lattice directly impacts how easily the compound can be dissolved. Stronger bonds mean higher lattice energy, resisting the disruption required for dissolution.
The Role of Polar Solvents in Dissolving Ionic Compounds
The solubility of an ionic compound is largely determined by the solvent's ability to overcome the strong electrostatic forces holding the ions together in the crystal lattice. Polar solvents, like water, possess molecules with a significant difference in electronegativity between their atoms. This leads to a partial positive charge on one end of the molecule (δ+) and a partial negative charge on the other (δ−).
Water, being the most common polar solvent, is exceptionally good at dissolving many ionic compounds. The partially charged ends of water molecules interact strongly with the ions in the ionic lattice. This interaction is called hydration, where water molecules surround and effectively shield the ions, weakening the attractive forces holding the lattice together.
The Hydration Process:
- Ion-dipole interaction: The partially negative oxygen atoms in water molecules are attracted to the positively charged cations, while the partially positive hydrogen atoms are attracted to the negatively charged anions.
- Solvation: This attraction overcomes the lattice energy, causing the ions to break away from the crystal lattice.
- Dispersion: The hydrated ions become surrounded by water molecules, preventing them from re-aggregating and remaining dispersed in the solution.
Factors Influencing the Solubility of Ionic Compounds
Several key factors besides the polarity of the solvent affect the solubility of ionic compounds:
1. Lattice Energy: The Strength of the Ionic Bond
As mentioned earlier, lattice energy is the energy required to separate one mole of a solid ionic compound into its gaseous ions. High lattice energy indicates strong ionic bonds, leading to lower solubility. This is because more energy is required to overcome the strong attractive forces holding the ions together. Smaller ions with higher charges generally have higher lattice energies and thus lower solubility.
2. Hydration Enthalpy: The Energy of Ion-Solvent Interaction
Hydration enthalpy is the energy released when one mole of gaseous ions is dissolved in water. High hydration enthalpy contributes to higher solubility because it provides the energy needed to overcome the lattice energy. Smaller ions with higher charge densities typically have higher hydration enthalpies.
3. Entropy: The Measure of Disorder
Dissolving an ionic compound increases the entropy (disorder) of the system. The highly ordered crystal lattice becomes a more disordered solution. This increase in entropy favors the dissolution process and contributes to solubility.
4. Temperature: Its Impact on Solubility
Temperature affects the solubility of ionic compounds differently depending on whether the dissolution process is endothermic (absorbs heat) or exothermic (releases heat). Generally, increasing temperature increases the solubility of most ionic compounds as it provides more energy to overcome the lattice energy. However, in some cases, the solubility may decrease with increasing temperature.
5. Common Ion Effect: The Presence of Similar Ions
The common ion effect describes the decrease in solubility of a sparingly soluble ionic compound when a common ion is added to the solution. The presence of additional ions of the same charge reduces the driving force for dissolution, leading to precipitation of the sparingly soluble salt.
6. Size and Charge of Ions: A Critical Interplay
The size and charge of ions significantly impact both lattice energy and hydration enthalpy. Smaller ions with higher charges experience stronger electrostatic attractions (higher lattice energy) and stronger ion-dipole interactions (higher hydration enthalpy). The interplay between these two factors determines the overall solubility. For example, smaller, highly charged ions like Al³⁺ have high lattice energies and high hydration enthalpies, leading to complex solubility behavior.
Exceptions and Complexities: When Ionic Compounds Don't Dissolve Easily
While many ionic compounds readily dissolve in water, several factors can lead to exceptions to this general rule:
- Very high lattice energy: Compounds with extremely strong ionic bonds (e.g., some metal oxides and sulfides) may have lattice energies exceeding the energy gained from hydration, leading to low solubility.
- Covalent character: Some ionic compounds exhibit significant covalent character, which weakens the ion-dipole interactions and reduces solubility.
- Formation of insoluble precipitates: When mixing two solutions containing ions that can form an insoluble ionic compound, a precipitate will form, demonstrating low solubility of that particular combination.
- Complex ion formation: In some cases, the addition of certain ligands can form complex ions with the metal cation, increasing the solubility of the compound.
Conclusion: Solubility - A Complex Dance of Forces
The solubility of ionic compounds is not a simple yes or no answer. It's a complex interplay of various factors, including lattice energy, hydration enthalpy, entropy, temperature, common ion effect, and the size and charge of ions. While many ionic compounds exhibit high solubility in polar solvents like water due to strong ion-dipole interactions, exceptions exist due to the complex interplay of these factors. Understanding these principles is crucial for predicting and manipulating the solubility of ionic compounds in various applications, from chemical reactions to environmental science and materials science. Further research into these nuanced aspects continues to refine our understanding of this fundamental aspect of chemistry.
Latest Posts
Latest Posts
-
How To Graph X 2 2
Mar 17, 2025
-
What Is 70 Inches In Cm
Mar 17, 2025
-
How Many Degrees Fahrenheit Is One Degree Celsius
Mar 17, 2025
-
1 8 As A Percent And Decimal
Mar 17, 2025
-
The Sum Of The Protons And Neutrons In An Atom
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Do Ionic Bonds Have High Solubility . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
