Do Ionic Bonds Dissolve In Water
listenit
Mar 20, 2025 · 5 min read
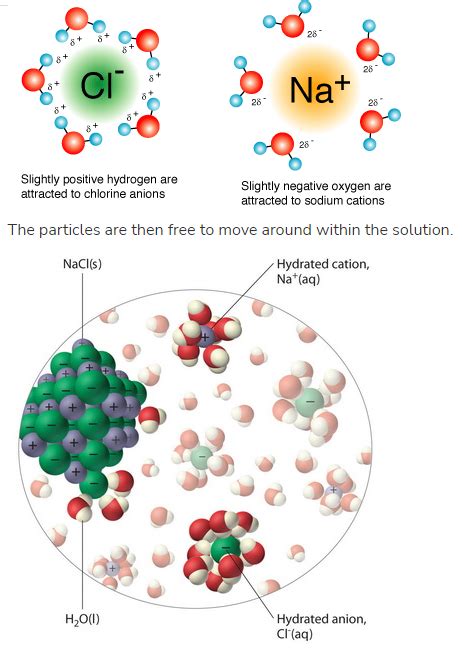
Table of Contents
Do Ionic Bonds Dissolve in Water? A Deep Dive into Solubility
The question of whether ionic bonds dissolve in water is a fundamental concept in chemistry with far-reaching implications across various fields, from biology to materials science. While the simple answer is often "yes," the reality is far more nuanced. This article will delve into the intricacies of ionic solubility in water, exploring the underlying principles, influencing factors, and exceptions to the rule.
Understanding Ionic Bonds and Their Polar Nature
Before examining solubility, it's crucial to understand the nature of ionic bonds. Ionic bonds form when one atom donates one or more electrons to another atom, resulting in the formation of ions – positively charged cations and negatively charged anions. This transfer creates a strong electrostatic attraction between these oppositely charged species, holding them together in a crystal lattice structure. These lattices are characteristically rigid and often have high melting and boiling points.
The key to understanding why many ionic compounds dissolve in water lies in the polarity of water molecules. Water (H₂O) is a polar molecule, meaning it has a slightly positive end (near the hydrogen atoms) and a slightly negative end (near the oxygen atom). This polarity arises from the difference in electronegativity between oxygen and hydrogen. Oxygen is more electronegative, meaning it attracts electrons more strongly, resulting in a partial negative charge on the oxygen and partial positive charges on the hydrogens.
The Solvation Process: How Water Dissolves Ionic Compounds
When an ionic compound is added to water, the polar water molecules interact with the ions in the crystal lattice. This interaction, known as solvation (or hydration when the solvent is water), is the driving force behind dissolution. The slightly negative oxygen atoms in water molecules are attracted to the positively charged cations, while the slightly positive hydrogen atoms are attracted to the negatively charged anions.
This attraction overcomes the electrostatic forces holding the ions together in the crystal lattice. Water molecules surround individual ions, effectively shielding them from each other and pulling them away from the lattice. This process is depicted as the hydration shells forming around individual ions, stabilizing them in solution.
Energetics of Dissolution: A Balancing Act
The dissolution of an ionic compound in water is a process governed by energy changes. Two key factors are involved:
-
Lattice energy: This is the energy required to break apart the ionic crystal lattice. It's a measure of the strength of the electrostatic attraction between the ions. High lattice energy means a strong lattice and lower solubility.
-
Hydration enthalpy: This is the energy released when water molecules surround the ions. A high hydration enthalpy favors dissolution because it provides the energy needed to overcome the lattice energy.
The overall solubility of an ionic compound is determined by the balance between these two energetic factors. If the hydration enthalpy is greater than the lattice energy, the dissolution process is exothermic (releases heat) and favored, resulting in high solubility. If the lattice energy is greater, the process is endothermic (absorbs heat) and less favored, resulting in lower solubility.
Factors Affecting the Solubility of Ionic Compounds
Several factors influence the solubility of ionic compounds in water beyond the simple lattice energy versus hydration enthalpy balance:
1. Charge Density of Ions:
The higher the charge density of the ions (charge divided by ionic radius), the stronger the attraction between the ions and the more difficult it is to separate them. Highly charged ions (e.g., Al³⁺, PO₄³⁻) tend to have lower solubility than singly charged ions (e.g., Na⁺, Cl⁻).
2. Ionic Radius:
Smaller ions have higher charge density and thus stronger interactions, leading to lower solubility. Larger ions experience weaker interactions and higher solubility.
3. Temperature:
The effect of temperature on solubility is complex and depends on whether the dissolution process is exothermic or endothermic. For most ionic compounds, solubility increases with temperature because the increased kinetic energy helps overcome the lattice energy. However, in some cases, solubility might decrease with increasing temperature.
4. Pressure:
Pressure has a negligible effect on the solubility of solids in liquids, including ionic compounds in water.
Exceptions to the Rule: Insoluble Ionic Compounds
While many ionic compounds dissolve readily in water, some are practically insoluble. This is often due to exceptionally strong lattice energies that outweigh the hydration enthalpy. Examples include many metal sulfides (e.g., PbS, CuS) and carbonates (e.g., CaCO₃). These compounds exhibit very low solubility in water.
Applications and Importance
The understanding of ionic solubility in water is crucial in numerous applications:
-
Medicine: Drug solubility is a critical factor in drug design and delivery. Understanding how ionic drugs dissolve in bodily fluids is essential for effective absorption and bioavailability.
-
Environmental Science: The solubility of ionic compounds dictates their behavior in natural water systems, influencing water quality and pollution control strategies.
-
Agriculture: The solubility of fertilizers, many of which are ionic compounds, directly affects nutrient uptake by plants.
-
Chemical Engineering: Solubility is a vital consideration in many industrial processes, including the design of separation techniques and the formulation of various products.
-
Geology: The solubility of minerals in groundwater influences weathering processes and the formation of caves and other geological formations.
Conclusion: A Complex Phenomenon
The solubility of ionic compounds in water is not a simple yes or no answer. It's a complex process determined by the interplay of multiple factors, primarily the balance between lattice energy and hydration enthalpy. While many ionic compounds readily dissolve in water due to the strong interaction between polar water molecules and the constituent ions, exceptions exist where the strong lattice energy prevents significant dissolution. Understanding these nuances is crucial across numerous scientific and technological disciplines. Further research continues to refine our understanding of these intricate interactions, leading to advancements in various fields.
Latest Posts
Latest Posts
-
What Is The Fraction Of 4 5
Mar 21, 2025
-
What Is 33 As A Fraction
Mar 21, 2025
-
What Is Half Of 2 1 3
Mar 21, 2025
-
What Element Has The Largest Ionization Energy
Mar 21, 2025
-
Is Reacts With Water A Physical Or Chemical Property
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Do Ionic Bonds Dissolve In Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
