Do Gases Have A Definite Volume
listenit
Mar 15, 2025 · 6 min read
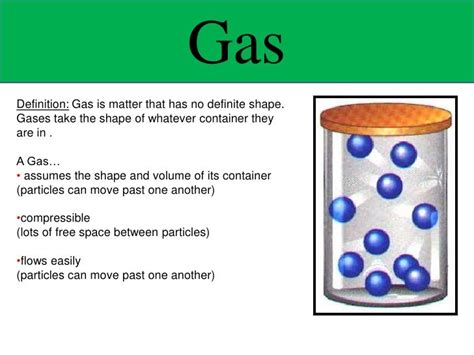
Table of Contents
Do Gases Have a Definite Volume? Exploring the Properties of Gases
The question of whether gases possess a definite volume is a fundamental concept in chemistry and physics. The short answer is no, gases do not have a definite volume. Unlike solids and liquids, which maintain a relatively fixed shape and volume, gases are highly compressible and readily expand to fill the available space. This characteristic stems from the unique nature of gas particles and their interactions. This comprehensive exploration delves deep into the properties of gases, explaining why they lack a definite volume and examining the factors that influence their behavior.
Understanding the Kinetic Molecular Theory of Gases
To grasp why gases don't have a definite volume, we must first understand the kinetic molecular theory (KMT) of gases. This theory provides a microscopic model explaining the macroscopic properties of gases. The KMT postulates the following:
- Gases consist of tiny particles (atoms or molecules) that are in constant, random motion. These particles are in continuous, chaotic movement, colliding with each other and the walls of their container.
- The volume of the gas particles themselves is negligible compared to the total volume of the gas. This means the particles occupy a relatively insignificant amount of space compared to the space between them.
- There are no significant attractive or repulsive forces between gas particles. The particles essentially act independently of one another, except during collisions.
- Collisions between gas particles and the container walls are perfectly elastic. This implies no loss of kinetic energy during collisions.
- The average kinetic energy of gas particles is directly proportional to the absolute temperature (in Kelvin). Higher temperatures mean faster-moving particles.
The Relationship Between Volume, Pressure, and Temperature
The behavior of gases is described by the ideal gas law, a mathematical equation that relates pressure (P), volume (V), temperature (T), and the number of moles (n) of gas:
PV = nRT
where R is the ideal gas constant.
This equation highlights the interdependence of these four variables. A change in one variable will necessarily lead to a change in at least one of the others, assuming the others remain constant. This directly relates to the undefined volume of gases. If you change the pressure or temperature, the volume will adjust accordingly. There is no inherent, fixed volume.
Influence of Pressure
Pressure is defined as the force exerted per unit area. In a gas, pressure results from the countless collisions of gas particles with the walls of their container. Increasing the pressure on a gas forces the particles closer together, thereby reducing the volume. Conversely, decreasing the pressure allows the gas to expand and occupy a larger volume. This demonstrates the compressibility of gases – a defining characteristic that solidifies their lack of a definite volume.
Influence of Temperature
Temperature is a measure of the average kinetic energy of gas particles. At higher temperatures, particles move faster and collide more frequently and forcefully. This leads to an increase in pressure unless the volume is allowed to expand. Conversely, at lower temperatures, particles move slower, resulting in lower pressure and a potential decrease in volume. The relationship between temperature and volume is directly proportional when pressure and the number of moles are held constant (Charles's Law).
Real Gases vs. Ideal Gases
The ideal gas law is a useful approximation, but it assumes that gases behave ideally – meaning they strictly adhere to the postulates of the KMT. In reality, most gases deviate from ideal behavior, especially at high pressures or low temperatures.
At high pressures, the volume of the gas particles becomes significant relative to the total volume. This is because the particles are forced so close together that their own volume can no longer be ignored.
At low temperatures, attractive forces between gas particles become more significant. These intermolecular forces cause the particles to clump together slightly, reducing the effective volume of the gas.
These deviations from ideal behavior are described by various equations of state, such as the van der Waals equation, which incorporate correction terms for intermolecular forces and particle volume. However, even with these corrections, the fundamental principle remains: gases do not possess a fixed, definite volume. They adapt their volume to the confines of their container and the prevailing conditions of pressure and temperature.
Examples Illustrating the Indefinite Volume of Gases
Several everyday examples showcase the indefinite volume of gases:
- Inflating a balloon: When you inflate a balloon, you are essentially adding gas molecules to the inside. The gas expands to fill the entire volume of the balloon, showcasing its ability to adapt its volume to its container.
- Filling a tire with air: Similar to the balloon, the air fills the tire completely. The pressure in the tire is determined by how much air is pumped in, which indirectly controls the volume of air present within the relatively fixed confines of the tire.
- Cooking with gases: Gases used for cooking, like propane or natural gas, expand to fill the space within the gas lines and appliances. They don't maintain a specific volume but adapt to the available space.
- Atmospheric pressure: The Earth's atmosphere is a mixture of gases. The atmosphere doesn't have a definite volume; it extends hundreds of kilometers into space, thinning out as altitude increases.
Practical Implications of the Indefinite Volume of Gases
The fact that gases do not have a definite volume has several practical implications:
- Storage and transportation: Gases need to be stored in containers capable of withstanding the pressure exerted by the gas. The volume of the container will directly influence the pressure and thus the safety of storage.
- Industrial processes: Many industrial processes involve the use of gases, and understanding their behavior is crucial for efficient operation and safety. Controlling pressure and temperature is key to managing gas volumes in these processes.
- Environmental science: Atmospheric gases play a critical role in climate change and air pollution. Understanding how gases behave in the atmosphere is essential for predicting and mitigating environmental problems.
- Medical applications: Gases are used in various medical applications, such as anesthesia and respiratory support. Precise control of gas pressure and volume is crucial in medical settings.
Conclusion: Gases Are Defined by Their Adaptability
In conclusion, the answer remains a resounding no. Gases do not possess a definite volume. Their volume is entirely dependent on the pressure, temperature, and the available space within their container. This property stems from the kinetic molecular theory and is fundamental to understanding the behavior of gases in various contexts, from everyday phenomena to complex industrial and scientific processes. The adaptability of gases, their ability to fill any available space, is a defining characteristic distinguishing them from solids and liquids, and underscores their unique properties.
Latest Posts
Latest Posts
-
Amino Acids Are The Monomers For
Mar 17, 2025
-
Why Cant Ions Pass Through The Membrane
Mar 17, 2025
-
What Is 170 Degrees C In Fahrenheit
Mar 17, 2025
-
175 Of What Number Is 42
Mar 17, 2025
-
What Percent Is 20 Out Of 50
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Do Gases Have A Definite Volume . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
