Amino Acids Are The Monomers For
listenit
Mar 17, 2025 · 7 min read
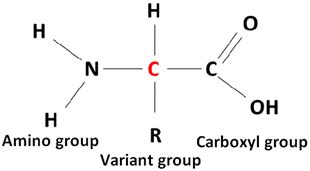
Table of Contents
Amino Acids: The Building Blocks of Life's Polymers
Amino acids are the fundamental monomers—the individual units—that combine to form the incredibly diverse and essential group of biological polymers known as proteins. Proteins are the workhorses of the cell, participating in virtually every cellular process imaginable. Understanding amino acids, therefore, is crucial to understanding the very essence of life itself. This article will delve into the structure, properties, and diverse roles of amino acids as the fundamental monomers for proteins, exploring their importance in various biological contexts.
The Structure of Amino Acids: A Universal Blueprint
The basic structure of an amino acid is surprisingly simple, yet this simplicity belies the immense complexity and functionality they achieve when linked together. Every amino acid consists of four key components attached to a central carbon atom, often called the alpha carbon:
- An amino group (-NH2): This is a nitrogen-containing group that acts as a base, accepting protons (H+) in certain environments.
- A carboxyl group (-COOH): This is an acidic group that can donate a proton (H+), acting as an acid.
- A hydrogen atom (-H): This is a simple hydrogen atom bonded to the alpha carbon.
- A side chain (R group): This is the variable component, unique to each of the twenty standard amino acids. The R group's properties—size, charge, polarity, etc.—dictate the amino acid's overall characteristics and its role in protein structure and function.
The Twenty Standard Amino Acids: A Diverse Cast
The twenty standard amino acids are categorized based on the properties of their R groups:
-
Nonpolar, aliphatic amino acids: These amino acids have hydrocarbon side chains that are hydrophobic (water-repelling). Examples include glycine, alanine, valine, leucine, isoleucine, and methionine. These amino acids often reside within the interior of proteins, away from the aqueous environment of the cell.
-
Aromatic amino acids: These possess aromatic rings in their side chains, contributing to their hydrophobic nature. Examples include phenylalanine, tyrosine, and tryptophan. They often play roles in protein interactions and light absorption.
-
Polar, uncharged amino acids: These amino acids have side chains with polar but uncharged functional groups, making them hydrophilic (water-attracting). Examples include serine, threonine, cysteine, asparagine, and glutamine. These amino acids often participate in hydrogen bonding within proteins and their interactions with water. Cysteine, uniquely, contains a thiol group (-SH) that can form disulfide bonds, crucial for stabilizing protein tertiary structure.
-
Positively charged (basic) amino acids: These amino acids have positively charged side chains at physiological pH. Examples include lysine, arginine, and histidine. Their positive charge contributes significantly to protein-protein interactions and enzymatic activity.
-
Negatively charged (acidic) amino acids: These amino acids possess negatively charged side chains at physiological pH. Examples include aspartic acid and glutamic acid. Their negative charges contribute to electrostatic interactions within proteins and their environment.
Peptide Bonds: Linking Amino Acids into Chains
Amino acids are linked together via peptide bonds, a type of covalent bond. This bond formation occurs between the carboxyl group (-COOH) of one amino acid and the amino group (-NH2) of another. This reaction releases a molecule of water (H2O), a process known as a dehydration reaction or condensation reaction. The resulting chain of amino acids is called a polypeptide.
The Peptide Bond's Properties: Dictating Protein Folding
The peptide bond possesses specific properties that significantly influence protein structure:
- Partial double bond character: The peptide bond exhibits a partial double bond character due to resonance, restricting rotation around the bond and affecting the overall conformation of the polypeptide chain.
- Planarity: The atoms involved in the peptide bond lie in a relatively planar configuration.
- Directionality: Polypeptide chains have directionality, with an N-terminus (the amino group end) and a C-terminus (the carboxyl group end).
From Polypeptide Chains to Functional Proteins: Levels of Protein Structure
The linear sequence of amino acids in a polypeptide chain is called the primary structure of a protein. However, the protein's function is determined by its three-dimensional structure, which arises through various levels of organization:
-
Secondary structure: This refers to local folding patterns within the polypeptide chain, primarily stabilized by hydrogen bonds between the backbone amide and carbonyl groups. Common secondary structures include alpha-helices (spiral-like structures) and beta-sheets (extended sheet-like structures).
-
Tertiary structure: This is the overall three-dimensional arrangement of a single polypeptide chain, determined by interactions between the side chains (R groups) of the amino acids. These interactions can include hydrophobic interactions, hydrogen bonds, ionic bonds, and disulfide bonds. The tertiary structure is crucial for the protein's function.
-
Quaternary structure: This refers to the arrangement of multiple polypeptide chains (subunits) to form a functional protein complex. Many proteins require multiple subunits to achieve their full functionality. Hemoglobin, for instance, is a tetramer—a protein composed of four subunits.
Amino Acids and Protein Function: A Diverse Range of Roles
Proteins perform an astonishing array of functions within living organisms, and the specific amino acid sequence directly dictates the protein's function. Some examples include:
-
Enzymes: These are biological catalysts that accelerate biochemical reactions. The active site of an enzyme, where the substrate binds, is precisely structured by the amino acid sequence.
-
Structural proteins: These proteins provide structural support and shape to cells and tissues. Collagen, a major component of connective tissue, is a prime example.
-
Transport proteins: These proteins facilitate the movement of molecules across cell membranes, such as hemoglobin, which transports oxygen in the blood.
-
Hormones: These proteins act as chemical messengers, regulating various physiological processes. Insulin, which regulates blood glucose levels, is a classic example.
-
Receptors: These proteins bind to specific molecules, initiating a cellular response. Cell surface receptors play crucial roles in cell signaling.
-
Antibodies: These proteins, part of the immune system, bind to antigens (foreign substances) and neutralize them.
-
Motor proteins: These proteins generate movement, such as myosin, which is responsible for muscle contraction.
Amino Acid Metabolism: Synthesis, Degradation, and Essential Amino Acids
The body can synthesize some amino acids (non-essential amino acids), while others must be obtained from the diet (essential amino acids). Essential amino acids cannot be synthesized by the body in sufficient quantities to meet its needs. The synthesis and degradation of amino acids are crucial metabolic processes involved in protein turnover, energy production, and the synthesis of other essential molecules.
Essential vs. Non-essential Amino Acids: Dietary Considerations
The classification of amino acids as essential or non-essential is crucial for nutrition. A balanced diet must provide all essential amino acids for proper protein synthesis and overall health. Deficiencies in essential amino acids can lead to various health problems, hindering protein synthesis and impairing bodily functions.
Beyond the Twenty Standard Amino Acids: Modified and Uncommon Amino Acids
While the twenty standard amino acids form the basis of most proteins, many other amino acids exist, either as modifications of the standard ones or as rare amino acids found in specific proteins. These modifications often play crucial roles in protein function and regulation. For example, post-translational modifications, such as phosphorylation or glycosylation, can alter an amino acid's properties and the function of the protein.
Conclusion: The Enduring Importance of Amino Acids
Amino acids are the fundamental building blocks of proteins, and understanding their structure, properties, and functions is essential to grasping the complexity and diversity of life. From the simplest single-celled organisms to the most complex multicellular beings, the twenty standard amino acids, along with their modified forms, form the basis of the incredible array of proteins that drive cellular processes, maintain structure, and enable life itself. Continued research into amino acids and their roles in various biological contexts will continue to yield critical insights into the fundamental mechanisms of life and potentially pave the way for advancements in medicine, biotechnology, and other fields. Their importance cannot be overstated.
Latest Posts
Latest Posts
-
Domain And Range Of X 1 X 2
Mar 17, 2025
-
100 Yards Equals How Many Feet
Mar 17, 2025
-
Words That Begin With Same Letter
Mar 17, 2025
-
Assume That The Function F Is A One To One Function
Mar 17, 2025
-
9 Is What Percent Of 50
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Amino Acids Are The Monomers For . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
