Do Covalent Bonds Dissolve In Water
listenit
Mar 16, 2025 · 5 min read
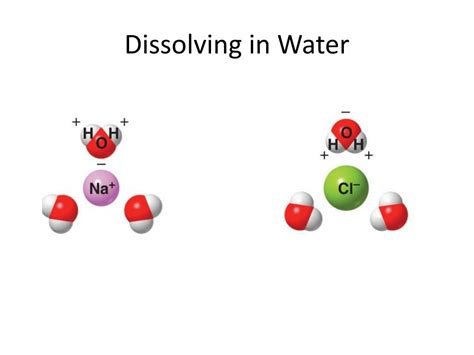
Table of Contents
Do Covalent Bonds Dissolve in Water? A Deep Dive into Solubility
The question of whether covalent bonds dissolve in water is a nuanced one, far from a simple yes or no. While the statement that "covalent compounds don't dissolve in water" is a common misconception often encountered in introductory chemistry, the reality is far more complex. The solubility of a covalent compound in water depends heavily on the nature of the covalent bonds, the molecular structure, and the intermolecular forces at play.
Understanding Covalent Bonds and Water
Before delving into the intricacies of solubility, let's establish a firm understanding of the key players: covalent bonds and water.
Covalent Bonds: Sharing is Caring
A covalent bond is formed when two atoms share one or more pairs of electrons to achieve a more stable electron configuration. This sharing creates a strong attractive force that holds the atoms together, forming a molecule. Examples of covalent compounds include methane (CH₄), glucose (C₆H₁₂O₆), and ethanol (C₂H₅OH).
Water: The Universal Solvent (With Caveats)
Water (H₂O) is a remarkably versatile solvent, often referred to as the "universal solvent." This is due to its polar nature. The oxygen atom in water is more electronegative than the hydrogen atoms, meaning it attracts the shared electrons more strongly. This creates a partial negative charge (δ-) on the oxygen and partial positive charges (δ+) on the hydrogens. This uneven distribution of charge creates a dipole moment, making water a polar molecule.
The Role of Polarity and Intermolecular Forces
The solubility of a covalent compound in water hinges primarily on the interaction between the water molecules and the molecules of the covalent compound. This interaction is governed by intermolecular forces, particularly dipole-dipole interactions and hydrogen bonds.
Polar Covalent Compounds: A Match Made in Heaven
Polar covalent compounds, like those containing oxygen, nitrogen, or fluorine atoms bonded to hydrogen or other electronegative atoms, exhibit a significant dipole moment. These polar molecules readily interact with the polar water molecules through dipole-dipole interactions. The positive end of one molecule attracts the negative end of another, creating a strong attractive force. The stronger these interactions are, the more soluble the compound will be in water.
Hydrogen bonding, a special type of dipole-dipole interaction, plays a crucial role in the solubility of many polar covalent compounds. It occurs when a hydrogen atom bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) is attracted to a lone pair of electrons on another electronegative atom in a different molecule. Hydrogen bonds are relatively strong and contribute significantly to the solubility of compounds like alcohols, carboxylic acids, and amines in water.
Nonpolar Covalent Compounds: Like Oil and Water
Nonpolar covalent compounds, such as hydrocarbons (compounds containing only carbon and hydrogen), have a symmetrical distribution of charge, resulting in a very small or zero dipole moment. They do not interact strongly with water molecules, leading to low solubility in water. The weak interactions between nonpolar molecules and water are mainly London dispersion forces, which are much weaker than dipole-dipole interactions or hydrogen bonds. This is why nonpolar substances like oil and grease do not dissolve in water.
Factors Affecting Solubility of Covalent Compounds
Several factors beyond polarity influence the solubility of covalent compounds in water:
Molecular Size and Shape
Larger molecules generally have lower solubility in water. This is because the increased surface area provides more sites for London dispersion forces to act, counteracting the effects of polar interactions with water. Similarly, the shape of the molecule affects its ability to interact with water molecules. A more compact, symmetrical shape often leads to lower solubility.
Functional Groups
The presence of specific functional groups within a molecule significantly impacts its solubility. Functional groups like hydroxyl (-OH), carboxyl (-COOH), amino (-NH₂), and carbonyl (C=O) are polar and increase the solubility of a compound in water by increasing the strength of dipole-dipole interactions and hydrogen bonding.
Temperature
Temperature affects the solubility of covalent compounds in water. Generally, increasing the temperature increases the solubility of solids and gases in water. The increased kinetic energy helps overcome the intermolecular forces holding the solute molecules together and promotes interaction with water molecules.
Pressure
Pressure primarily affects the solubility of gases in water. Increasing the pressure increases the solubility of gases, as it forces more gas molecules into solution. However, it has minimal impact on the solubility of solid covalent compounds.
Examples: Illustrating the Nuances
Let's consider some specific examples to highlight the complexities:
1. Glucose (C₆H₁₂O₆): Glucose is a polar covalent compound with multiple hydroxyl (-OH) groups, capable of forming numerous hydrogen bonds with water molecules. Therefore, glucose is highly soluble in water.
2. Ethanol (C₂H₅OH): Ethanol contains a hydroxyl group, allowing it to form hydrogen bonds with water. It's miscible with water, meaning it dissolves completely in all proportions.
3. Methane (CH₄): Methane is a nonpolar hydrocarbon with very weak London dispersion forces. It is essentially insoluble in water.
4. Oils and Fats: These are nonpolar compounds composed primarily of long hydrocarbon chains. Their insolubility in water is responsible for the separation of oil and water.
5. Acetic Acid (CH₃COOH): Acetic acid, a weak acid, possesses both a polar carboxyl group capable of hydrogen bonding and a nonpolar methyl group. The presence of the polar group makes it soluble in water, although not as completely as ethanol.
Conclusion: It's Complicated!
The question of whether covalent bonds dissolve in water is not a straightforward yes or no. The solubility of a covalent compound depends on the interplay of several factors, including the polarity of the molecule, its size and shape, the presence of functional groups, and intermolecular forces. While nonpolar covalent compounds generally exhibit low solubility in water, many polar covalent compounds readily dissolve due to strong interactions with water molecules. Understanding these complexities is vital for predicting and explaining the behavior of covalent compounds in aqueous solutions, across diverse scientific fields from chemistry and biology to environmental science and materials science. The key takeaway is that solubility is not solely determined by the presence of covalent bonds but rather by the intricate balance of forces between the solute and the solvent.
Latest Posts
Latest Posts
-
What Are The Rows On The Periodic Table Called
Mar 16, 2025
-
Words That Start With The Same Letter
Mar 16, 2025
-
What Is The Derivative Of 3 X
Mar 16, 2025
-
Least Common Multiple For 4 And 7
Mar 16, 2025
-
What Is The Derivative Of 3x
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about Do Covalent Bonds Dissolve In Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
