Difference Between Sn1 And Sn2 Reactions
listenit
Mar 21, 2025 · 6 min read
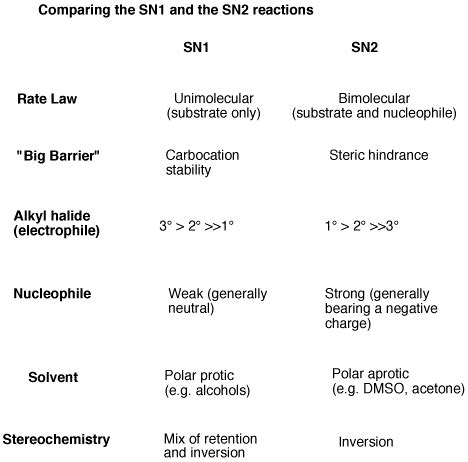
Table of Contents
- Difference Between Sn1 And Sn2 Reactions
- Table of Contents
- Unveiling the Differences: SN1 vs. SN2 Reactions in Organic Chemistry
- Understanding the Fundamentals: Nucleophiles and Leaving Groups
- SN2 Reactions: A Concerted Mechanism
- Key Characteristics of SN2 Reactions:
- SN1 Reactions: A Two-Step Mechanism
- Key Characteristics of SN1 Reactions:
- Comparing SN1 and SN2 Reactions: A Tabular Summary
- Factors Influencing SN1 vs. SN2 Reaction Pathways
- Applications and Significance
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Unveiling the Differences: SN1 vs. SN2 Reactions in Organic Chemistry
Organic chemistry, a cornerstone of chemistry, delves into the fascinating world of carbon-containing compounds and their reactions. Among the diverse reactions, nucleophilic substitutions (SN) stand out as fundamental processes. These reactions involve the replacement of a leaving group in a molecule by a nucleophile – a species with a lone pair of electrons seeking a positive charge. However, two prominent mechanisms govern these substitutions: SN1 and SN2. Understanding the key distinctions between these two mechanisms is crucial for predicting reaction outcomes and designing synthetic strategies. This comprehensive guide will meticulously dissect the differences between SN1 and SN2 reactions, covering their mechanisms, kinetics, stereochemistry, and factors influencing their preference.
Understanding the Fundamentals: Nucleophiles and Leaving Groups
Before delving into the specifics of SN1 and SN2 reactions, let's briefly define the key players:
-
Nucleophile (Nu⁻): A nucleophile is a species with a lone pair of electrons or a π bond that can donate electrons to form a new bond with an electron-deficient atom. Strong nucleophiles readily donate their electrons, while weak nucleophiles are less inclined to do so. Examples include hydroxide ion (OH⁻), cyanide ion (CN⁻), and halides (Cl⁻, Br⁻, I⁻). The strength of a nucleophile is influenced by factors like charge, electronegativity, steric hindrance, and solvent.
-
Leaving Group (LG): A leaving group is an atom or group of atoms that departs from the molecule during the reaction, taking with it a pair of electrons. Good leaving groups are weak bases, readily accepting the electron pair. Common examples include halides (Cl⁻, Br⁻, I⁻), water (H₂O), and tosylate (OTs). The stability of the leaving group greatly impacts the reaction rate.
SN2 Reactions: A Concerted Mechanism
SN2 (substitution nucleophilic bimolecular) reactions proceed through a concerted mechanism, meaning the bond breaking and bond formation occur simultaneously in a single step. This single-step process involves a transition state where the nucleophile approaches the substrate from the backside, opposite the leaving group. The nucleophile attacks the carbon atom bearing the leaving group, while simultaneously the leaving group departs.
Key Characteristics of SN2 Reactions:
-
Bimolecular: The rate of the reaction depends on the concentration of both the substrate and the nucleophile. The rate law is expressed as: Rate = k[substrate][nucleophile].
-
Concerted Mechanism: Bond breaking and bond formation occur simultaneously.
-
Backside Attack: The nucleophile attacks the carbon atom from the side opposite the leaving group, resulting in inversion of configuration (stereochemistry). This is often described as a "Walden inversion."
-
Steric Hindrance: Steric hindrance around the carbon atom bearing the leaving group significantly affects the reaction rate. Bulky groups hinder the approach of the nucleophile, slowing down the reaction. Methyl and primary halides are the most reactive in SN2 reactions, while tertiary halides are essentially unreactive.
-
Strong Nucleophiles: SN2 reactions favor strong nucleophiles, which readily attack the substrate.
-
Aprotic Solvents: Aprotic solvents, which lack O-H or N-H bonds, are preferred because they do not solvate the nucleophile, keeping it reactive. Examples include acetone, DMF, and DMSO.
SN1 Reactions: A Two-Step Mechanism
SN1 (substitution nucleophilic unimolecular) reactions occur in two distinct steps. First, the leaving group departs, forming a carbocation intermediate. Subsequently, the nucleophile attacks the carbocation, forming the final product.
Key Characteristics of SN1 Reactions:
-
Unimolecular: The rate-determining step involves only the substrate, and the rate law is: Rate = k[substrate].
-
Two-Step Mechanism: The reaction proceeds through a carbocation intermediate.
-
Carbocation Stability: The stability of the carbocation intermediate dictates the reaction rate. Tertiary carbocations are the most stable, followed by secondary, then primary. Methyl carbocations are exceptionally unstable and do not readily form.
-
Racemization: The carbocation intermediate is planar, allowing the nucleophile to attack from either side, leading to a racemic mixture of products (a 50:50 mixture of enantiomers) unless the leaving group departs from a chiral center already bonded to at least two different substituents. However the major product will have the inverted configuration.
-
Weak Nucleophiles: SN1 reactions can proceed with weak nucleophiles because the nucleophilic attack is a fast step that occurs after the rate-determining step (leaving group departure).
-
Protic Solvents: Protic solvents, which have O-H or N-H bonds, are often favored in SN1 reactions because they can stabilize the carbocation intermediate through solvation.
Comparing SN1 and SN2 Reactions: A Tabular Summary
| Feature | SN1 Reaction | SN2 Reaction |
|---|---|---|
| Mechanism | Two-step, carbocation intermediate | Concerted, single-step |
| Rate Law | Rate = k[substrate] | Rate = k[substrate][nucleophile] |
| Molecularity | Unimolecular | Bimolecular |
| Substrate | Tertiary > Secondary > Primary > Methyl | Methyl > Primary > Secondary > Tertiary |
| Nucleophile | Weak nucleophiles can be used | Strong nucleophiles are preferred |
| Leaving Group | Good leaving group required | Good leaving group required |
| Stereochemistry | Racemization (usually) | Inversion of configuration (Walden inversion) |
| Solvent | Protic solvents often favored | Aprotic solvents often favored |
| Steric Hindrance | Less sensitive to steric hindrance | Highly sensitive to steric hindrance |
Factors Influencing SN1 vs. SN2 Reaction Pathways
Several factors influence whether a reaction will favor an SN1 or SN2 mechanism:
-
Substrate Structure: Tertiary substrates favor SN1 reactions due to the stability of the resulting tertiary carbocation. Primary substrates favor SN2 reactions due to the reduced steric hindrance. Secondary substrates can undergo both SN1 and SN2 reactions, with the preference depending on the other factors.
-
Nucleophile Strength: Strong nucleophiles favor SN2 reactions, while weak nucleophiles favor SN1 reactions.
-
Leaving Group Ability: A good leaving group is essential for both SN1 and SN2 reactions, but the leaving group's ability affects the rates differently for the two mechanisms.
-
Solvent: Protic solvents stabilize the carbocation intermediate in SN1 reactions and solvate the nucleophile in SN2 reactions.
-
Temperature: Higher temperatures generally favor SN1 reactions because they provide the activation energy needed for carbocation formation.
Applications and Significance
The understanding of SN1 and SN2 reactions is vital in various fields:
-
Organic Synthesis: SN1 and SN2 reactions are cornerstones of organic synthesis, used to build complex molecules from simpler ones. Chemists carefully select reagents and conditions to favor the desired mechanism to control the stereochemistry and regiochemistry of the products.
-
Drug Discovery: SN1 and SN2 reactions are involved in the synthesis of numerous pharmaceuticals, where precise control of reaction pathways is critical.
-
Polymer Chemistry: SN1 and SN2 reactions play a role in the synthesis of various polymers, influencing their properties and characteristics.
-
Biochemical Processes: Analogous reactions to SN1 and SN2 occur in biological systems, including enzymatic reactions.
Conclusion
The distinction between SN1 and SN2 reactions lies in their mechanistic pathways, kinetics, and stereochemical outcomes. Understanding these differences is fundamental for predicting reaction products and designing efficient synthetic strategies in organic chemistry. By considering factors such as substrate structure, nucleophile strength, leaving group ability, and solvent effects, chemists can effectively control the reaction pathway and achieve the desired outcome. The applications of SN1 and SN2 reactions extend far beyond the confines of the laboratory, impacting various scientific disciplines and technological advancements. Therefore, a thorough grasp of these mechanisms is essential for anyone pursuing a career in chemistry or related fields.
Latest Posts
Latest Posts
-
Alliteration In Speech I Have A Dream
Mar 28, 2025
-
What Is An Example Of An Biotic Factor
Mar 28, 2025
-
What Is A 40 Out Of 60
Mar 28, 2025
-
What Are The Three Steps In The Water Cycle
Mar 28, 2025
-
What Is The Value Of R 2 3 4 5
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Difference Between Sn1 And Sn2 Reactions . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
