Copper Ii Nitrate With Sodium Hydroxide
listenit
Apr 01, 2025 · 5 min read
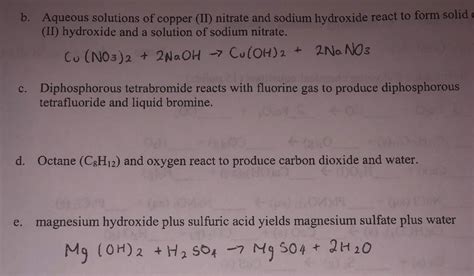
Table of Contents
Copper(II) Nitrate and Sodium Hydroxide: A Comprehensive Exploration
The reaction between copper(II) nitrate and sodium hydroxide is a classic example of a double displacement reaction, often used in chemistry demonstrations and experiments to illustrate precipitation reactions. This article delves deep into the intricacies of this reaction, exploring its chemical equation, the properties of the reactants and products, the procedure for carrying out the reaction, safety precautions, applications, and potential extensions for further study. Understanding this seemingly simple reaction provides a solid foundation for grasping more complex chemical principles.
The Chemical Reaction: A Detailed Look
The reaction between copper(II) nitrate (Cu(NO₃)₂) and sodium hydroxide (NaOH) is a double displacement reaction, also known as a metathesis reaction. This means that the positive and negative ions of the two reactants switch partners to form two new compounds. The balanced chemical equation for this reaction is:
Cu(NO₃)₂(aq) + 2NaOH(aq) → Cu(OH)₂(s) + 2NaNO₃(aq)
This equation tells us that one mole of aqueous copper(II) nitrate reacts with two moles of aqueous sodium hydroxide to produce one mole of solid copper(II) hydroxide and two moles of aqueous sodium nitrate. The "(aq)" denotes an aqueous solution (dissolved in water), while "(s)" indicates a solid precipitate.
Understanding the Reactants
-
Copper(II) Nitrate (Cu(NO₃)₂): This is a blue crystalline solid that is highly soluble in water, forming a bright blue solution. It's an ionic compound composed of copper(II) cations (Cu²⁺) and nitrate anions (NO₃⁻). Copper(II) nitrate is a common source of copper(II) ions in laboratory settings and has various industrial applications.
-
Sodium Hydroxide (NaOH): Also known as caustic soda or lye, sodium hydroxide is a white crystalline solid that is highly soluble in water, producing a strongly alkaline solution. It's a strong base, readily dissociating into sodium cations (Na⁺) and hydroxide anions (OH⁻) in aqueous solution. Sodium hydroxide has extensive industrial uses, including in the production of soap, paper, and textiles.
Understanding the Products
-
Copper(II) Hydroxide (Cu(OH)₂): This is a light blue, gelatinous precipitate that forms as a result of the reaction. It is sparingly soluble in water. The formation of this precipitate is the driving force behind this reaction.
-
Sodium Nitrate (NaNO₃): This is a colorless, crystalline solid that is highly soluble in water. It's an ionic compound composed of sodium cations (Na⁺) and nitrate anions (NO₃⁻). Sodium nitrate is a relatively inert compound and is commonly used as a fertilizer and food preservative.
The Precipitation Reaction: A Step-by-Step Guide
Performing this reaction in a laboratory setting is straightforward but requires careful attention to safety precautions (discussed later). Here's a step-by-step procedure:
-
Prepare the solutions: Prepare aqueous solutions of copper(II) nitrate and sodium hydroxide of known concentrations. The exact concentrations will depend on the desired scale of the experiment.
-
Combine the solutions: Slowly add the sodium hydroxide solution to the copper(II) nitrate solution while stirring gently. The addition should be done dropwise to control the rate of precipitation.
-
Observe the precipitation: As the sodium hydroxide solution is added, a light blue precipitate of copper(II) hydroxide will begin to form. The solution will change from a clear blue to a cloudy, light blue suspension.
-
Allow settling: Once the addition is complete, allow the mixture to settle. The copper(II) hydroxide precipitate will settle at the bottom of the container, leaving a relatively clear supernatant liquid containing sodium nitrate.
-
Separation (optional): The precipitate can be separated from the supernatant liquid using techniques like decantation or filtration.
Safety Precautions: Prioritizing Safety
When working with copper(II) nitrate and sodium hydroxide, it's crucial to prioritize safety:
-
Eye protection: Always wear safety goggles to protect your eyes from splashes.
-
Gloves: Wear appropriate chemical-resistant gloves to prevent skin contact with the solutions.
-
Ventilation: Conduct the experiment in a well-ventilated area or under a fume hood to minimize exposure to fumes. Sodium hydroxide solutions are corrosive, and copper(II) nitrate can be an irritant.
-
Disposal: Dispose of the waste solutions properly according to your institution's guidelines. Copper(II) hydroxide is not considered highly hazardous, but proper waste disposal practices should still be followed.
Applications and Further Exploration
The reaction between copper(II) nitrate and sodium hydroxide, while seemingly simple, has several applications and can serve as a springboard for further exploration:
-
Qualitative Analysis: This reaction is frequently used in qualitative analysis to identify the presence of copper(II) ions. The formation of the characteristic light blue precipitate confirms the presence of copper(II).
-
Synthesis of Copper(II) Oxide: Heating the copper(II) hydroxide precipitate results in the decomposition to form copper(II) oxide (CuO), a black solid. This provides a pathway for the synthesis of another important copper compound. The equation is:
Cu(OH)₂(s) → CuO(s) + H₂O(g)
-
Exploring Equilibrium: The reaction's equilibrium can be investigated by varying concentrations and temperatures, demonstrating Le Chatelier's principle.
-
Stoichiometry Calculations: The reaction provides an excellent platform for practicing stoichiometry calculations, allowing students to determine the limiting reactant, theoretical yield, and percent yield.
-
Ksp Determination: The solubility of copper(II) hydroxide is low, allowing for the determination of its solubility product constant (Ksp) through experimentation.
-
Spectrophotometry: The intensity of the blue color of the copper(II) nitrate solution can be measured using a spectrophotometer, and the change in absorbance during the reaction can be monitored.
Conclusion: A Reaction with Rich Applications
The reaction between copper(II) nitrate and sodium hydroxide is a fundamental chemical reaction with broad implications. Beyond its simplicity, it serves as a valuable tool for illustrating key concepts in chemistry, from precipitation reactions and stoichiometry to equilibrium and qualitative analysis. By understanding this reaction thoroughly, students and researchers alike can develop a deeper appreciation for the interconnectedness of chemical phenomena and the power of simple reactions to reveal complex principles. Its versatility makes it an ideal subject for various experimental investigations, offering opportunities for both qualitative and quantitative explorations in chemical processes. The safety precautions mentioned are crucial for safe and responsible experimentation, emphasizing the importance of responsible chemical handling practices.
Latest Posts
Latest Posts
-
Write Each Equation In Standard Form Using Integers
Apr 02, 2025
-
What Is 24 In A Fraction
Apr 02, 2025
-
Moment Of Inertia Of A Hollow Cylinder
Apr 02, 2025
-
Whats The Square Root Of 125
Apr 02, 2025
-
How Is Ecosystem Different From Community
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Copper Ii Nitrate With Sodium Hydroxide . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
