Can An Ionic Compound Conduct Electricity
listenit
Mar 15, 2025 · 6 min read
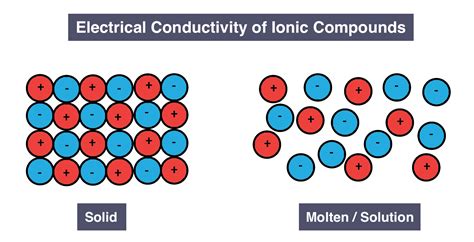
Table of Contents
Can an Ionic Compound Conduct Electricity? A Deep Dive into Conductivity
The ability of a substance to conduct electricity is a fundamental property with significant implications in various fields, from electronics to biology. Understanding this property, especially in the context of ionic compounds, requires a closer look at the nature of chemical bonding and charge mobility. This article will delve into the intricacies of electrical conductivity in ionic compounds, exploring the factors influencing this behavior and clarifying common misconceptions.
What is Electrical Conductivity?
Electrical conductivity refers to a material's ability to allow the flow of electric current. This flow is essentially the movement of charged particles, which can be electrons or ions. Materials are broadly classified into conductors, insulators, and semiconductors based on their conductivity. Conductors readily allow current flow, insulators strongly resist it, and semiconductors exhibit conductivity that falls between these extremes and is often influenced by external factors like temperature or light.
Ionic Compounds: A Structure-Property Relationship
Ionic compounds are formed through the electrostatic attraction between oppositely charged ions. A positively charged ion (cation) and a negatively charged ion (anion) are held together by strong ionic bonds, creating a crystalline structure. This structure is characterized by a regular, repeating arrangement of ions, with cations and anions alternating in a lattice. The key to understanding their electrical conductivity lies in the mobility of these ions.
Conductivity in the Solid State: A Rigid Structure
In the solid state, ionic compounds generally do not conduct electricity. This is because the ions are tightly held in their lattice positions by strong electrostatic forces. They are essentially locked in place and cannot move freely to carry an electric current. While the ions possess charge, their immobility prevents them from contributing to conductivity. Applying an external electric field cannot easily overcome the strong electrostatic forces holding the ions within the crystal lattice. Therefore, solid ionic compounds act as insulators.
The Exception: Certain Ionic Solids with Defects
While the general rule is that solid ionic compounds are insulators, there are exceptions. Crystalline defects, such as vacancies or interstitial ions within the lattice structure, can provide pathways for ion migration. These imperfections disrupt the perfect arrangement of ions, allowing a small degree of ion mobility. However, the conductivity in such cases remains relatively low compared to true conductors.
Conductivity in the Molten State: A Sea of Ions
The situation changes dramatically when an ionic compound is melted. The melting process breaks down the rigid crystal lattice, freeing the ions from their fixed positions. In the molten state, the ions become mobile, forming a fluid of charged particles. This mobility allows the ions to respond to an applied electric field, resulting in the flow of electric current. Therefore, molten ionic compounds are good conductors of electricity. The higher the temperature, the greater the kinetic energy of the ions, and consequently, the higher the conductivity.
Conductivity in Aqueous Solution: Solvation and Mobility
When an ionic compound is dissolved in water (or another polar solvent), the water molecules surround the ions, a process called solvation or hydration. This process weakens the electrostatic attractions between the ions and separates them, effectively freeing them to move independently. The presence of water molecules reduces the electrostatic forces of attraction between the ions, making them mobile and facilitating conduction.
The separated ions are now free to migrate under the influence of an applied electric field, leading to the conduction of electricity. The conductivity of an aqueous solution of an ionic compound depends on several factors:
- Concentration: Higher concentration means more ions are available to carry the current, resulting in higher conductivity.
- Nature of the ions: The size and charge of the ions affect their mobility. Smaller and more highly charged ions generally have higher mobility and contribute more significantly to conductivity.
- Temperature: Higher temperatures increase the kinetic energy of the ions, enhancing their mobility and conductivity.
- Solvent: The nature of the solvent plays a role in the solvation process and the mobility of the ions. Polar solvents, like water, are more effective in dissolving and solvating ionic compounds compared to nonpolar solvents.
Strong vs. Weak Electrolytes
The extent to which an ionic compound dissociates into ions in a solution determines its classification as a strong or weak electrolyte. Strong electrolytes completely dissociate into ions, resulting in high conductivity. Weak electrolytes only partially dissociate, leading to lower conductivity. This difference arises from the strength of the ionic bonds and the interactions between the ions and the solvent molecules.
Applications of Ionic Conductivity
The conductivity of ionic compounds, particularly in their molten or aqueous states, has numerous applications in various fields.
- Electroplating: Electroplating uses an electric current to deposit a thin layer of metal onto a surface. Molten salts containing the desired metal ions are often used in this process.
- Electrorefining: Similar to electroplating, electrorefining utilizes an electric current to purify metals. The impure metal is dissolved in a molten ionic compound, and the pure metal is deposited on a cathode.
- Batteries: Batteries rely on the movement of ions between electrodes to generate electric current. Many battery systems utilize ionic compounds in their electrolytes.
- Fuel Cells: Fuel cells convert chemical energy into electrical energy using ionic conductors as electrolytes. These electrolytes facilitate the transport of ions between the electrodes.
- Corrosion: The conductivity of ionic solutions plays a significant role in corrosion processes. The movement of ions in electrolytes contributes to the degradation of metallic materials.
- Medical Applications: Electrolytes, which are solutions containing ionic compounds, are crucial for maintaining fluid balance and proper bodily functions.
Misconceptions about Ionic Conductivity
Several misconceptions often surround the conductivity of ionic compounds:
- All salts conduct electricity: While many salts conduct electricity in solution, solid salts generally do not.
- Conductivity is only about electrons: While electron flow is crucial in metallic conduction, ionic conductivity involves the movement of ions.
- Higher concentration always means higher conductivity: While higher concentration generally leads to increased conductivity, other factors, such as ion mobility and solvent effects, also play a crucial role.
Conclusion
The electrical conductivity of ionic compounds is a complex phenomenon intricately linked to their structure and the mobility of their constituent ions. While solid ionic compounds generally act as insulators due to the immobility of their ions, their molten state and aqueous solutions exhibit significant conductivity due to the free movement of ions. This behavior has crucial implications in various applications, from electroplating and batteries to biological processes. A thorough understanding of the factors influencing ionic conductivity is essential for designing and optimizing technologies that utilize these properties. The relationship between the structure of ionic compounds, the state of matter they exist in, and their conductivity provides a fascinating illustration of how macroscopic properties arise from the microscopic interactions of charged particles.
Latest Posts
Latest Posts
-
Log X 3 Log X 1
Mar 15, 2025
-
8x 4 4x 3 4 6x 4 4
Mar 15, 2025
-
What Do The Arrows On A Food Chain Represent
Mar 15, 2025
-
Is Salt An Element Compound Or Mixture
Mar 15, 2025
-
How Many Millimeters Are In One Meter
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Can An Ionic Compound Conduct Electricity . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
