Boiling Water Chemical Or Physical Change
listenit
Mar 16, 2025 · 5 min read
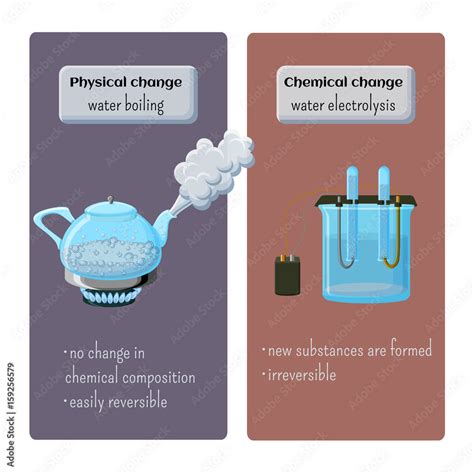
Table of Contents
Boiling Water: A Physical Change, Not Chemical
The question of whether boiling water represents a chemical or physical change is a fundamental one in science, often encountered in introductory chemistry courses. The answer, simply put, is physical. But understanding why it's a physical change requires a deeper dive into the concepts of physical and chemical changes, the properties of water, and the process of boiling itself. This article will thoroughly explore the topic, providing a comprehensive understanding backed by scientific principles and addressing common misconceptions.
Understanding Physical and Chemical Changes
Before we delve into the specifics of boiling water, let's establish a clear understanding of the distinction between physical and chemical changes.
Physical Changes
A physical change alters the form or appearance of a substance but does not change its chemical composition. The molecules of the substance remain the same; they might be rearranged or separated, but their fundamental structure remains unaltered. Examples include:
- Changes of state: Melting ice, boiling water, freezing liquid, sublimation (solid to gas), deposition (gas to solid). These transitions involve changes in energy levels and molecular arrangement, but the water molecules themselves remain H₂O.
- Dissolving: Salt dissolving in water is a physical change. The salt molecules are dispersed in the water, but they retain their chemical identity. Evaporation of the water would leave the salt behind, unchanged.
- Cutting, grinding, or crushing: These actions change the shape and size of a substance but do not alter its chemical makeup.
Chemical Changes
A chemical change, also known as a chemical reaction, involves the formation of new substances with different chemical properties. The original substance(s) undergo a transformation, resulting in molecules with different arrangements of atoms. Examples include:
- Burning: Combustion reactions involve the reaction of a substance with oxygen, producing new compounds like carbon dioxide and water.
- Rusting: The oxidation of iron in the presence of oxygen and water creates iron oxide, a new substance with different properties.
- Cooking: Many cooking processes involve chemical changes, such as the denaturation of proteins in eggs when they are cooked.
The Process of Boiling Water: A Detailed Look
Let's examine the boiling of water step-by-step to solidify its classification as a physical change.
Molecular Behavior in Liquid Water
Water (H₂O) exists as a liquid due to the strong hydrogen bonds between its molecules. These bonds are relatively weak compared to covalent bonds (the bonds within the H₂O molecule itself), but they are strong enough to hold the molecules together in a loosely structured arrangement. The molecules are constantly moving and colliding, but the hydrogen bonds prevent them from completely escaping each other's influence.
Adding Heat: Increasing Kinetic Energy
When heat is applied to liquid water, the molecules absorb energy. This energy increases the kinetic energy of the molecules, causing them to move faster and more vigorously. The hydrogen bonds are constantly breaking and reforming as the molecules jostle against each other.
Reaching the Boiling Point: Overcoming Intermolecular Forces
As the temperature increases, the kinetic energy of the water molecules eventually surpasses the strength of the hydrogen bonds holding them together. At the boiling point (100°C or 212°F at standard atmospheric pressure), enough energy is present to overcome these intermolecular forces.
Vaporization: The Transition to Gas
At the boiling point, the water molecules gain sufficient kinetic energy to break free from the liquid phase and escape as water vapor (steam). This process is called vaporization or boiling. The water molecules themselves remain intact; they are simply transitioning from a more closely packed liquid state to a more dispersed gaseous state. Crucially, the chemical formula remains H₂O.
Condensation: Reversing the Process
The process of boiling can be reversed through condensation. When water vapor cools, the molecules lose kinetic energy. The weaker intermolecular forces (in this case, van der Waals forces) between the water molecules become significant, allowing them to re-aggregate and form liquid water once again. This transition, like boiling, is a physical change.
Addressing Common Misconceptions
Several misconceptions surround the boiling of water and its classification as a physical change. Let's address some of them:
Misconception 1: Bubbles Indicate a Chemical Reaction
Many people associate the formation of bubbles during boiling with a chemical reaction. However, the bubbles are simply water vapor, a change in the physical state of water, not the formation of a new chemical substance. The bubbles are composed of water molecules in the gaseous phase.
Misconception 2: Changes in Taste or Appearance Mean Chemical Change
While boiling may alter the taste (e.g., removing volatile compounds) or appearance (e.g., evaporating some dissolved minerals) of the water, these are still physical changes. The underlying chemical composition of water (H₂O) remains unaffected. The changes in taste or appearance are related to the removal of other substances dissolved within the water, not changes in the water molecule itself.
Misconception 3: Energy Change Implies Chemical Change
The fact that energy (heat) is required to boil water doesn't automatically signify a chemical change. Many physical changes involve energy changes. For instance, melting ice also requires energy input, yet it is a physical change. The energy is used to overcome intermolecular forces, not to break or form chemical bonds.
Beyond Boiling: Other Physical Changes of Water
The boiling of water is just one example of water undergoing a physical change. Other examples include:
- Melting: The transition from ice (solid) to liquid water.
- Freezing: The transition from liquid water to ice.
- Sublimation: The direct transition from ice (solid) to water vapor (gas), bypassing the liquid phase. This often occurs at low temperatures and pressures.
- Deposition: The direct transition from water vapor (gas) to ice (solid).
Conclusion: Boiling Water Remains H₂O
Boiling water is unequivocally a physical change. While the state of the water changes from liquid to gas, the chemical composition—H₂O—remains unchanged. The process involves a change in energy and molecular arrangement, but no new substances are formed. Understanding this distinction is crucial for grasping fundamental concepts in chemistry and scientific observation in general. The consistent chemical identity of the water molecule throughout the boiling process solidifies its classification as a purely physical transformation. The key takeaway is to focus on whether the fundamental chemical structure of the substance changes—in the case of boiling water, it does not.
Latest Posts
Latest Posts
-
Lowest Common Multiple Of 6 And 15
Mar 16, 2025
-
Graph Of X 2 Y 2 4
Mar 16, 2025
-
Whats The Square Root Of 196
Mar 16, 2025
-
15 Is What Percent Of 38
Mar 16, 2025
-
Is 2 3 More Than 3 4 Cup
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about Boiling Water Chemical Or Physical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
