Balance Equation C2h6 O2 Co2 H2o
listenit
May 09, 2025 · 5 min read
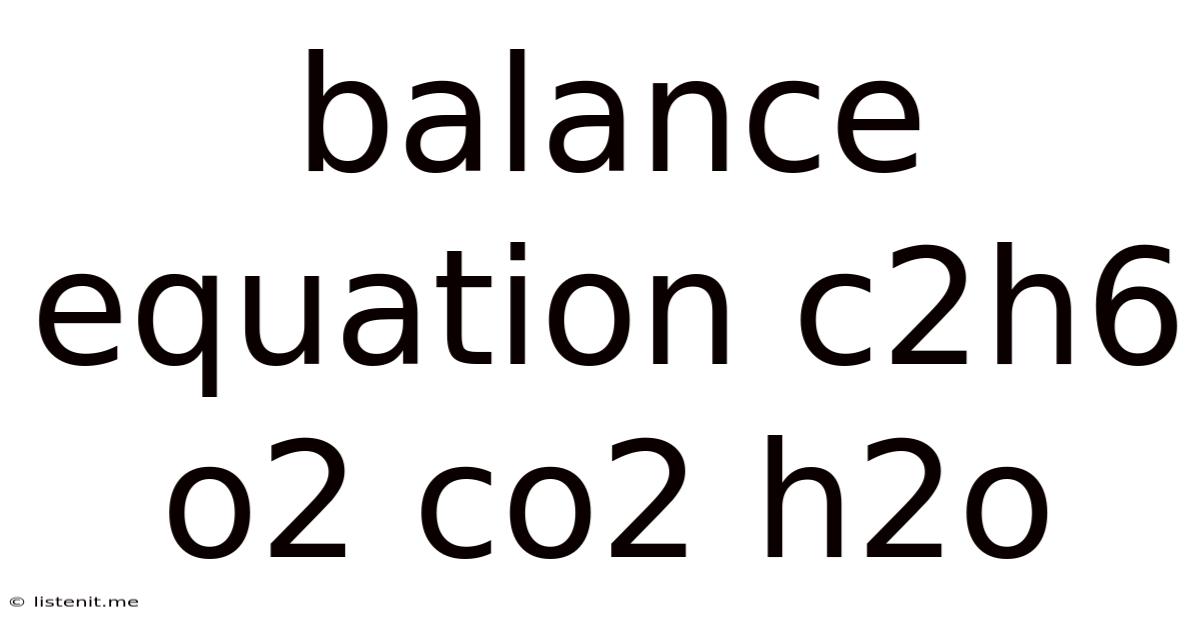
Table of Contents
Balancing the Combustion Equation: C₂H₆ + O₂ → CO₂ + H₂O
The complete combustion of ethane (C₂H₆) in the presence of oxygen (O₂) to produce carbon dioxide (CO₂) and water (H₂O) is a fundamental chemical reaction with significant implications in various fields, from understanding energy production to analyzing atmospheric chemistry. This article delves deep into balancing the chemical equation for this reaction, exploring the steps involved, the importance of stoichiometry, and the practical applications of this balanced equation. We'll also touch upon the implications of incomplete combustion and the environmental considerations surrounding ethane combustion.
Understanding the Combustion Reaction
Before diving into the balancing process, let's establish a clear understanding of the reaction itself. The combustion of ethane is an exothermic reaction, meaning it releases heat. This is why ethane is considered a fuel, and its combustion is utilized for energy generation. The general reaction can be represented as:
C₂H₆ + O₂ → CO₂ + H₂O
This equation, however, is unbalanced. Balancing chemical equations is crucial because it adheres to the law of conservation of mass, stating that matter cannot be created or destroyed in a chemical reaction. The number of atoms of each element must be the same on both sides of the equation.
Balancing the Equation: A Step-by-Step Guide
Balancing chemical equations is a systematic process. Here's a step-by-step guide to balance the combustion of ethane:
Step 1: Count the atoms.
Begin by counting the number of atoms of each element on both the reactant (left) and product (right) sides of the equation:
- Reactants: 2 Carbon (C) atoms, 6 Hydrogen (H) atoms, and 2 Oxygen (O) atoms.
- Products: 1 Carbon (C) atom, 2 Hydrogen (H) atoms, and 3 Oxygen (O) atoms.
Step 2: Balance the carbon atoms.
We have 2 carbon atoms on the reactant side and only 1 on the product side. To balance this, we add a coefficient of 2 in front of CO₂:
C₂H₆ + O₂ → 2CO₂ + H₂O
Now, we have 2 carbon atoms on both sides.
Step 3: Balance the hydrogen atoms.
There are 6 hydrogen atoms on the reactant side and only 2 on the product side. To balance, we add a coefficient of 3 in front of H₂O:
C₂H₆ + O₂ → 2CO₂ + 3H₂O
Now, we have 6 hydrogen atoms on both sides.
Step 4: Balance the oxygen atoms.
This is often the most challenging step. After balancing carbon and hydrogen, we now have 7 oxygen atoms on the product side (4 from 2CO₂ and 3 from 3H₂O) and only 2 on the reactant side. To balance this, we add a coefficient of 7/2 in front of O₂:
C₂H₆ + 7/2O₂ → 2CO₂ + 3H₂O
This balances the oxygen atoms.
Step 5: Convert to whole numbers (if necessary).
While the equation is balanced, it's generally preferred to use whole number coefficients. To achieve this, we multiply the entire equation by 2:
2C₂H₆ + 7O₂ → 4CO₂ + 6H₂O
Now the equation is completely balanced with whole-number coefficients. We have 4 carbon atoms, 12 hydrogen atoms, and 14 oxygen atoms on both sides of the equation.
Stoichiometry and its Implications
The balanced equation, 2C₂H₆ + 7O₂ → 4CO₂ + 6H₂O, provides crucial stoichiometric information. Stoichiometry is the quantitative relationship between reactants and products in a chemical reaction. This balanced equation tells us:
- Mole Ratios: 2 moles of ethane react with 7 moles of oxygen to produce 4 moles of carbon dioxide and 6 moles of water.
- Mass Ratios: Using the molar masses of each substance, we can calculate the mass ratios involved in the reaction, allowing for precise calculations in various applications.
- Volume Ratios: For gases under the same conditions of temperature and pressure, the volume ratios are the same as the mole ratios.
This information is vital for various practical applications, including:
- Fuel Efficiency Calculations: Determining the amount of oxygen required for complete combustion of a given amount of ethane and the amount of CO₂ produced.
- Combustion Engine Design: Optimizing the air-fuel ratio in combustion engines for maximum efficiency and minimum emissions.
- Industrial Processes: Controlling reaction conditions in industrial processes involving ethane combustion.
Incomplete Combustion: A Different Scenario
The balanced equation we derived represents complete combustion. In reality, incomplete combustion can occur, especially under conditions with limited oxygen supply. Incomplete combustion produces carbon monoxide (CO) and/or soot (carbon, C) instead of or in addition to carbon dioxide. The equations for incomplete combustion are more complex and vary depending on the oxygen availability. For example, one possible incomplete combustion reaction is:
2C₂H₆ + 5O₂ → 4CO + 6H₂O
This reaction produces carbon monoxide, a highly toxic gas. Incomplete combustion is less efficient in terms of energy release and poses significant environmental and health risks.
Environmental Considerations
The combustion of ethane, while a significant source of energy, also contributes to environmental concerns:
- Greenhouse Gas Emissions: The production of carbon dioxide (CO₂) contributes to the greenhouse effect and global warming.
- Air Pollution: Incomplete combustion leads to the emission of harmful pollutants like carbon monoxide and particulate matter, impacting air quality and human health.
- Climate Change: The release of greenhouse gases from ethane combustion contributes to climate change and its associated effects.
Therefore, mitigating the environmental impact of ethane combustion involves strategies such as:
- Improving Combustion Efficiency: Ensuring complete combustion to minimize CO and soot emissions.
- Carbon Capture and Storage: Developing technologies to capture and store CO₂ released during combustion.
- Transitioning to Renewable Energy Sources: Reducing reliance on fossil fuels like ethane by transitioning to cleaner energy sources.
Conclusion
Balancing the chemical equation for the combustion of ethane is a fundamental step in understanding this crucial reaction. The balanced equation, 2C₂H₆ + 7O₂ → 4CO₂ + 6H₂O, provides critical stoichiometric information for various applications, from fuel efficiency calculations to industrial process control. However, it's vital to consider the implications of incomplete combustion and its environmental consequences. Striving for complete combustion and exploring alternative energy sources are essential to mitigate the environmental impact of ethane combustion and ensure a sustainable future. The understanding of this seemingly simple chemical reaction has profound implications for energy production, environmental protection, and our overall understanding of chemical processes.
Latest Posts
Latest Posts
-
How To Find Diameter When Given The Circumference
May 09, 2025
-
Find The Missing Side To The Nearest Tenth
May 09, 2025
-
A Human Diploid Cell Has How Many Chromosomes
May 09, 2025
-
4 Divided By 1 3 In Fraction Form
May 09, 2025
-
Sodium Chloride Silver Nitrate Balanced Equation
May 09, 2025
Related Post
Thank you for visiting our website which covers about Balance Equation C2h6 O2 Co2 H2o . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.