An Increase In The Temperature Of A Solution Usually
listenit
Mar 16, 2025 · 6 min read
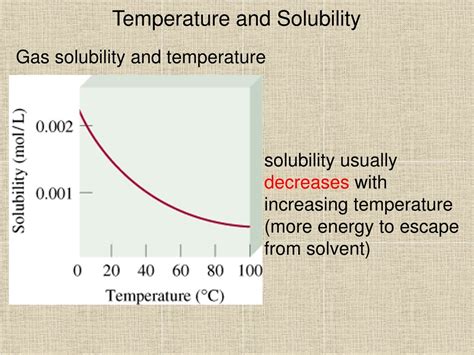
Table of Contents
An Increase in the Temperature of a Solution: Causes, Effects, and Applications
An increase in the temperature of a solution is a common phenomenon with far-reaching consequences across various scientific disciplines and everyday life. Understanding the causes, effects, and applications of this temperature change is crucial for numerous fields, from chemistry and physics to environmental science and engineering. This article delves into the intricacies of this seemingly simple process, exploring its underlying mechanisms and practical implications.
Causes of an Increase in Solution Temperature
Several factors can contribute to a rise in the temperature of a solution. These can be broadly categorized into:
1. Exothermic Reactions: The Heat of Reaction
Many chemical reactions release energy in the form of heat. These are known as exothermic reactions. When an exothermic reaction occurs in a solution, the released heat increases the solution's temperature. The magnitude of the temperature increase depends on several factors, including:
- The enthalpy change (ΔH) of the reaction: A more negative ΔH indicates a more exothermic reaction and a larger temperature increase.
- The concentration of reactants: Higher concentrations generally lead to a greater heat release and temperature rise.
- The heat capacity of the solution: Solutions with lower heat capacities experience larger temperature increases for the same amount of heat added. The heat capacity is a measure of a substance's resistance to temperature change.
- Heat loss to the surroundings: The rate of heat transfer to the surroundings affects the final temperature of the solution. Insulating the container can minimize heat loss and maximize the temperature increase.
Examples of Exothermic Reactions leading to increased solution temperature:
- Acid-base neutralization reactions: Mixing a strong acid and a strong base releases a significant amount of heat.
- Combustion reactions: Burning fuels in a solution (e.g., dissolving a flammable substance) generates substantial heat.
- Many metal displacement reactions: The replacement of one metal by another in a solution often results in an exothermic reaction and a temperature increase.
2. Heat Transfer from External Sources: Direct Heating
Direct application of heat to a solution, such as through a Bunsen burner, hot plate, or immersion heater, directly increases its temperature. The rate of temperature increase depends on factors like:
- The heat source's power: A more powerful heat source leads to a faster temperature increase.
- The surface area of the container: A larger surface area allows for more efficient heat transfer.
- The solution's volume: A larger volume of solution requires more energy to increase its temperature.
- Stirring: Stirring ensures uniform heating and prevents localized overheating.
3. Dissolution of Certain Substances: Heat of Solution
The process of dissolving a substance in a solvent can either release or absorb heat. When the dissolution process releases heat, it's called an exothermic dissolution, leading to a temperature increase. This heat is known as the heat of solution. The magnitude of the heat of solution varies greatly depending on the solute and solvent involved.
Examples of substances with exothermic dissolution:
- Strong acids and bases: Dissolving concentrated strong acids or bases in water often leads to a significant temperature increase.
- Certain salts: Many salts, like sodium hydroxide (NaOH) and potassium hydroxide (KOH), release heat upon dissolving.
4. Adiabatic Compression: Compressing Gases in Solution
In some cases, compressing a gas dissolved in a liquid can increase the solution's temperature. This process is adiabatic, meaning there's no heat exchange with the surroundings. The compression work is converted into internal energy, resulting in a temperature increase. This is less common in everyday scenarios but is relevant in certain industrial processes.
Effects of an Increased Solution Temperature
An increase in the solution's temperature has several significant effects, impacting its physical and chemical properties:
1. Increased Kinetic Energy of Molecules: Enhanced Reactivity
Higher temperatures lead to increased kinetic energy of the molecules within the solution. This increased kinetic energy leads to:
- Increased reaction rates: Molecules collide more frequently and with greater energy, increasing the probability of successful collisions and accelerating chemical reactions. This is described by the Arrhenius equation.
- Enhanced solubility of certain substances: The solubility of many solids and gases increases with temperature.
- Changes in solution density: The density of most liquids decreases with increasing temperature due to increased molecular motion and expansion.
2. Phase Transitions: Boiling and Evaporation
At sufficiently high temperatures, the solution may undergo phase transitions:
- Boiling: If the solution's temperature reaches its boiling point, it transitions into the gaseous phase.
- Evaporation: Even below the boiling point, some molecules possess sufficient kinetic energy to escape the liquid phase, leading to evaporation and cooling of the remaining solution.
3. Changes in Solution Properties: Viscosity and Surface Tension
Temperature changes affect several physical properties of solutions:
- Viscosity: The viscosity (resistance to flow) of most liquids decreases with increasing temperature.
- Surface tension: Surface tension generally decreases with increasing temperature.
4. Chemical Equilibrium Shifts: Le Chatelier's Principle
For reversible reactions in solution, an increase in temperature shifts the equilibrium position according to Le Chatelier's principle. If the forward reaction is endothermic (absorbs heat), increasing the temperature favors the forward reaction. If the forward reaction is exothermic (releases heat), increasing the temperature favors the reverse reaction.
Applications of Controlled Temperature Increase in Solutions
The ability to control and manipulate the temperature of solutions is essential across many fields:
1. Chemical Synthesis and Reactions: Optimizing Reaction Conditions
In chemical synthesis, controlling the solution temperature is critical for optimizing reaction yields and selectivity. Exothermic reactions often require cooling to prevent runaway reactions or decomposition. Endothermic reactions may require heating to ensure sufficient reaction rates.
2. Analytical Chemistry: Solubility and Separation Techniques
Temperature control is crucial in analytical chemistry for various techniques, including:
- Solubility studies: Determining the solubility of substances at different temperatures.
- Recrystallization: Purifying substances by dissolving them in a hot solvent and allowing them to recrystallize upon cooling.
- Chromatography: Temperature control is important in chromatographic separations to optimize resolution and efficiency.
3. Biological and Biochemical Applications: Enzyme Activity and Cell Culture
Temperature significantly affects biological processes:
- Enzyme activity: Enzyme activity is highly temperature-dependent, with optimal activity usually at a specific temperature range. Increasing the temperature beyond this range can lead to enzyme denaturation.
- Cell culture: Cell cultures require carefully controlled temperature conditions for optimal growth and function.
4. Industrial Processes: Heat Transfer and Chemical Processing
Many industrial processes involve heating or cooling solutions:
- Heat exchangers: Used to transfer heat between different fluids, including solutions.
- Chemical reactors: The temperature in chemical reactors is carefully controlled to optimize reaction efficiency and safety.
- Food processing: Heating and cooling solutions are used in various food processing steps, such as pasteurization and sterilization.
5. Environmental Science: Water Quality and Pollution Control
Temperature changes in water bodies affect aquatic life and water quality. Understanding the causes and effects of temperature changes is crucial for managing water resources and mitigating pollution.
Conclusion
An increase in the temperature of a solution is a fundamental phenomenon with significant implications in diverse areas of science and technology. Understanding the underlying causes, effects, and applications of this temperature change is essential for numerous applications, from optimizing chemical reactions and analyzing samples to maintaining biological systems and managing industrial processes. The ability to control and manipulate solution temperature provides a powerful tool for scientists and engineers to achieve their objectives effectively and safely. Further research and development in this area will undoubtedly lead to new advancements and applications across various scientific disciplines.
Latest Posts
Latest Posts
-
Nouns That Start With A U
Mar 16, 2025
-
Half A Pound Is How Many Ounces
Mar 16, 2025
-
What Is A Compound Represented By
Mar 16, 2025
-
What Is 23 Out Of 30
Mar 16, 2025
-
What Makes Up The Rungs Of Dna
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about An Increase In The Temperature Of A Solution Usually . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
