A Horizontal Row Of Elements In The Periodic Table
listenit
Mar 26, 2025 · 7 min read
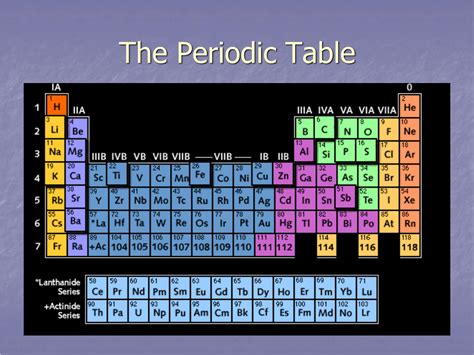
Table of Contents
A Horizontal Row of Elements: Exploring the Periodic Table's Periods
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic number and recurring chemical properties. A fundamental aspect of its structure is the arrangement of elements into horizontal rows, known as periods. Understanding the properties and trends within a period is crucial to grasping the fundamental principles of chemistry and predicting the behavior of elements. This article delves deep into the intricacies of a horizontal row in the periodic table, exploring the underlying trends, exceptions, and the significance of their arrangement.
What Defines a Period?
Each period represents a principal energy level or shell in an atom. As we move across a period from left to right, the atomic number increases sequentially, indicating an increase in the number of protons and electrons. The electrons fill the atomic orbitals within a given energy level, leading to predictable variations in chemical and physical properties. The number of elements in each period is determined by the number of electrons that can occupy the subshells of that principal energy level. The first period, for example, only contains two elements, hydrogen (H) and helium (He), as the first energy level (n=1) only possesses one s-subshell, accommodating a maximum of two electrons.
Significance of Electronic Configuration
The electronic configuration of an element is the key to understanding its position and properties within a period. This configuration dictates how many electrons are present in each orbital and subshell. Elements within the same period have the same highest principal quantum number (n). This means their valence electrons, the outermost electrons involved in chemical bonding, occupy the same principal energy level. The number of valence electrons directly impacts the chemical reactivity and bonding characteristics of an element.
Trends Across a Period: A Systematic Exploration
As we traverse a period, several key properties exhibit distinct trends:
1. Atomic Radius: A Gradual Decrease
Atomic radius refers to the size of an atom. Across a period, the atomic radius generally decreases. This is because, while additional electrons are added to the same energy level, the nuclear charge (number of protons) also increases. The increased positive charge pulls the electrons closer to the nucleus, resulting in a smaller atomic radius. This trend is especially pronounced across the transition metal series.
2. Ionization Energy: A General Increase
Ionization energy is the energy required to remove an electron from a neutral atom. Across a period, ionization energy generally increases. This is a direct consequence of the increasing nuclear charge. As the nucleus holds the electrons more tightly, it becomes increasingly difficult to remove an electron, hence the higher ionization energy. There can be minor irregularities due to electron shielding effects and electron-electron repulsions, particularly with elements having half-filled or fully-filled subshells.
3. Electronegativity: A Rise to the Right
Electronegativity measures the ability of an atom to attract electrons in a chemical bond. Across a period, electronegativity generally increases. This is because of the same reasons as ionization energy: the increasing nuclear charge enhances the atom's pull on shared electrons in a bond. Fluorine (F), positioned at the far right of the second period, is the most electronegative element.
4. Electron Affinity: A Complex Pattern
Electron affinity is the energy change that occurs when an atom gains an electron. The trend across a period is less straightforward compared to ionization energy and electronegativity. While there’s a general increase in electron affinity across a period (with exceptions), the trend isn't as consistent due to factors like electron-electron repulsions and the stability of half-filled and fully-filled subshells.
5. Metallic Character: A Decline to Non-Metallic Nature
Metallic character describes the properties typically associated with metals, such as conductivity, malleability, and ductility. Across a period, metallic character generally decreases. This is because the increasing nuclear charge results in a stronger attraction between the nucleus and the valence electrons, making it harder for the atoms to lose electrons and form positive ions, a characteristic feature of metals. Hence, elements on the right side of a period typically exhibit non-metallic behavior.
Exceptions to the Trends: Unpredictability in the Periodic Table
While the trends discussed above provide a general framework for understanding the properties of elements within a period, it’s crucial to acknowledge exceptions. These exceptions arise due to several factors:
- Electron Shielding: Inner electrons partially shield the outer electrons from the full effect of the nuclear charge. The extent of shielding varies depending on the subshells involved.
- Electron-Electron Repulsions: Repulsions between electrons in the same subshell can affect the overall energy levels and influence atomic properties.
- Half-filled and Fully-filled Subshells: Atoms with half-filled or fully-filled subshells exhibit enhanced stability, leading to deviations from expected trends. For example, the ionization energy of nitrogen (N) is higher than oxygen (O), despite the higher nuclear charge of oxygen. This is due to the extra stability associated with nitrogen's half-filled p-subshell.
Periods and Chemical Reactivity: Linking Structure to Behavior
The position of an element within a period directly influences its chemical reactivity. Elements on the left side of a period (alkali and alkaline earth metals) readily lose electrons to achieve a stable electron configuration, typically resembling the noble gas in the previous period. These elements are highly reactive. In contrast, elements on the right side of a period (halogens and noble gases) have a strong tendency to gain or share electrons to complete their outermost shell, achieving the noble gas configuration. Halogens are highly reactive nonmetals, while noble gases are exceptionally inert due to their full valence shells.
The Significance of Periods in Chemical Reactions
The arrangement of elements within periods plays a vital role in understanding and predicting the outcomes of chemical reactions. For example, the reactivity of alkali metals (Group 1) increases as we move down a group (column) due to increasing atomic size and decreasing ionization energy. This increased reactivity directly stems from the easier loss of valence electrons. Conversely, the reactivity of halogens (Group 17) decreases down the group due to the increasing atomic size and decreasing electron affinity. The larger the size of the halogen atom, the less readily it gains an electron.
Exploring Specific Periods: A Case Study Approach
Let's examine a few periods in detail to further illustrate these trends:
The Second Period (Li-Ne): A Miniature of Periodic Trends
This period demonstrates the fundamental periodic trends clearly. The atomic radius decreases from lithium (Li) to neon (Ne). Ionization energy, electronegativity, and electron affinity generally increase across the period. Metallic character decreases, transitioning from lithium's metallic nature to neon's inert gaseous state.
The Third Period (Na-Ar): Observing Similarities and Differences
The third period exhibits similar trends to the second period. However, the effects of increased shielding and electron-electron repulsions become more apparent. The differences in electronegativity and reactivity between elements in the third period and their counterparts in the second period highlight the gradual increase in atomic size and electron shielding down the group.
The Sixth Period (Cs-Rn): Lanthanides and the Impact on Properties
The sixth period is unique due to the inclusion of the lanthanides (rare earth elements). Their presence influences the electronic configuration and atomic properties of the elements that follow. The gradual filling of the 4f orbitals affects the atomic size and reactivity of elements in the sixth period.
Conclusion: Understanding Periods for a Deeper Chemical Insight
Understanding the trends and characteristics of elements within a period is fundamental to grasping the core principles of chemistry. The periodic table's horizontal rows, or periods, offer a structured framework for comprehending the interconnectedness of elements and their diverse properties. By analyzing the gradual changes in atomic radius, ionization energy, electronegativity, and other properties across a period, we gain valuable insights into the behavior of elements and can predict their reactivity in chemical reactions. The exceptions and deviations from expected trends add to the complexity and richness of the periodic table, underscoring the need for a comprehensive understanding of electronic configuration and atomic structure for a truly profound appreciation of chemistry. Furthermore, understanding periodic trends becomes crucial for applications in various fields, including material science, biochemistry, and drug discovery, highlighting the pivotal role of periods in shaping our understanding of the chemical world.
Latest Posts
Latest Posts
-
Two Most Abundant Elements In Earths Crust
Mar 29, 2025
-
Where Is Most Of The Freshwater Found
Mar 29, 2025
-
The Top Number In A Fraction Is Called
Mar 29, 2025
-
What Is The Least Common Multiple Of 4 6 9
Mar 29, 2025
-
Whats The Reciprocal Of 2 3
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about A Horizontal Row Of Elements In The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
