A Bond In Which Electrons Are Shared Equally
listenit
Mar 16, 2025 · 6 min read
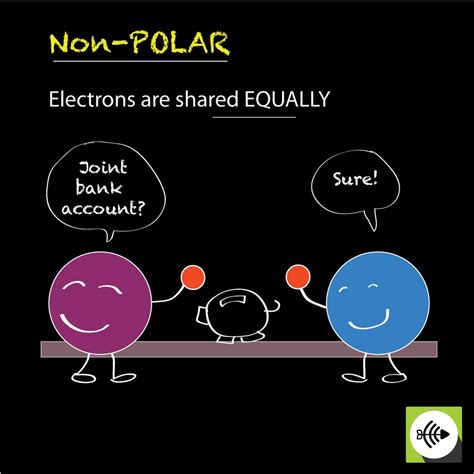
Table of Contents
A Bond in Which Electrons are Shared Equally: Exploring Nonpolar Covalent Bonds
A fundamental concept in chemistry is the chemical bond, the force that holds atoms together to form molecules and compounds. Among the various types of chemical bonds, the nonpolar covalent bond stands out as a quintessential example of equal electron sharing. Understanding this type of bond is crucial to grasping the properties and behaviors of countless substances in the world around us. This article will delve deep into the characteristics of nonpolar covalent bonds, exploring their formation, properties, examples, and the subtle nuances that differentiate them from other bond types.
What is a Nonpolar Covalent Bond?
A nonpolar covalent bond is a type of chemical bond where two atoms share electrons equally. This equal sharing occurs when the atoms involved have similar or identical electronegativity values. Electronegativity is a measure of an atom's ability to attract electrons towards itself in a chemical bond. When electronegativity values are close or identical, neither atom exerts a significantly stronger pull on the shared electrons, resulting in an even distribution of electronic charge. This contrasts sharply with polar covalent bonds, where electrons are shared unequally due to a difference in electronegativity between the atoms.
The Role of Electronegativity
The concept of electronegativity is pivotal in determining the type of bond formed between two atoms. The Pauling scale is commonly used to represent electronegativity values. Elements with similar electronegativity values, typically found within the same group or period, are more likely to form nonpolar covalent bonds. For example, a bond between two identical atoms (e.g., H-H in H₂ or O=O in O₂) will always be nonpolar because the electronegativity difference is zero.
Formation of Nonpolar Covalent Bonds
Nonpolar covalent bonds form when two atoms, each needing to complete their valence electron shells, share electrons. The sharing allows both atoms to achieve a stable electron configuration, usually resembling the nearest noble gas. This stable configuration is often referred to as an octet, satisfying the rule of eight electrons in the outermost shell for most elements. Hydrogen, however, only needs two electrons to achieve a stable configuration, satisfying the duet rule.
Let's consider the formation of a hydrogen molecule (H₂). Each hydrogen atom has one electron in its outermost shell. To achieve a stable duet, each atom shares its single electron with the other. This sharing creates a bond where the two electrons are equally shared between the two hydrogen nuclei.
Properties of Substances with Nonpolar Covalent Bonds
Substances formed primarily through nonpolar covalent bonds exhibit several characteristic properties:
-
Low Melting and Boiling Points: The relatively weak intermolecular forces (London Dispersion Forces) between nonpolar molecules require less energy to overcome, leading to lower melting and boiling points compared to substances with ionic or polar covalent bonds.
-
Poor Solubility in Water: Nonpolar substances generally do not dissolve well in water (a polar solvent). This is because "like dissolves like," and the nonpolar molecules have limited interactions with the polar water molecules.
-
Good Solubility in Nonpolar Solvents: Conversely, nonpolar substances readily dissolve in nonpolar solvents, such as hexane or benzene, due to the similar intermolecular forces.
-
Non-conductors of Electricity: Nonpolar covalent compounds do not conduct electricity because they lack freely moving charged particles (ions or electrons).
-
Often Gases or Liquids at Room Temperature: The weak intermolecular forces result in many nonpolar covalent compounds existing as gases or liquids at room temperature.
Examples of Nonpolar Covalent Bonds
Numerous molecules are held together primarily by nonpolar covalent bonds. Here are some prominent examples:
-
Diatomic Molecules: The diatomic elements—hydrogen (H₂), oxygen (O₂), nitrogen (N₂), fluorine (F₂), chlorine (Cl₂), bromine (Br₂), and iodine (I₂)—all exhibit nonpolar covalent bonding because they consist of two atoms of the same element.
-
Hydrocarbons: Hydrocarbons are organic compounds composed solely of carbon and hydrogen atoms. The C-H bonds and C-C bonds within hydrocarbons are essentially nonpolar due to the relatively small electronegativity difference between carbon and hydrogen. Examples include methane (CH₄), ethane (C₂H₆), and benzene (C₆H₆).
-
Aliphatic and Alicyclic Compounds: These classes of organic compounds consist of carbon chains or rings with attached hydrogen atoms and possibly other nonpolar groups. The bonds within these structures are largely nonpolar.
Distinguishing Nonpolar Covalent Bonds from Other Bond Types
It is crucial to distinguish nonpolar covalent bonds from other types of chemical bonds:
-
Polar Covalent Bonds: In polar covalent bonds, electrons are shared unequally due to a significant difference in electronegativity between the atoms. This results in a partial positive charge (δ+) on the less electronegative atom and a partial negative charge (δ-) on the more electronegative atom. Water (H₂O) is a classic example of a molecule with polar covalent bonds.
-
Ionic Bonds: Ionic bonds involve the complete transfer of electrons from one atom to another, resulting in the formation of ions (cations and anions). The electrostatic attraction between these oppositely charged ions forms the ionic bond. Sodium chloride (NaCl) is a typical example of an ionic compound.
-
Metallic Bonds: Metallic bonds occur in metals where valence electrons are delocalized and shared among a lattice of metal atoms. This creates a "sea" of electrons responsible for the characteristic properties of metals like conductivity and malleability.
Subtleties and Exceptions in Nonpolar Covalent Bonding
While the concept of equal electron sharing is central to nonpolar covalent bonds, some subtleties and exceptions exist:
-
No perfectly nonpolar bond: In reality, perfectly nonpolar bonds are rare. Even in diatomic molecules like H₂, a slight difference in electronegativity might exist due to subtle variations in electron distribution. However, the difference is often negligible and can be considered nonpolar for practical purposes.
-
Influence of molecular geometry: The overall polarity of a molecule depends not only on the individual bond polarities but also on the molecule's three-dimensional geometry. Even if a molecule contains polar bonds, its symmetrical arrangement can lead to cancellation of the dipole moments, resulting in a nonpolar molecule. For example, carbon dioxide (CO₂) has polar C=O bonds, but its linear geometry leads to a nonpolar molecule.
Conclusion
Nonpolar covalent bonds are fundamental to understanding the structure and properties of a vast array of substances. The equal sharing of electrons, driven by similar electronegativity values, leads to distinct characteristics such as low melting points, poor water solubility, and non-conductivity. While exceptions and subtleties exist, understanding the principles behind nonpolar covalent bonds provides crucial insights into the fascinating world of chemical bonding and molecular interactions. Further exploration into the intricacies of chemical bonding opens doors to deeper understanding in various scientific fields, from materials science to biological chemistry. The seemingly simple concept of equal electron sharing underlies a complex and fascinating aspect of the natural world.
Latest Posts
Latest Posts
-
Is Boiling Water A Physical Change Or Chemical Change
Mar 16, 2025
-
What Percent Of 70 Is 35
Mar 16, 2025
-
What Is The Lcm Of 3 And 9
Mar 16, 2025
-
Where Does Transcription Take Place In A Prokaryotic Cell
Mar 16, 2025
-
Is Water A Biotic Or Abiotic Factor
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about A Bond In Which Electrons Are Shared Equally . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
