Is Boiling Water A Physical Change Or Chemical Change
listenit
Mar 16, 2025 · 5 min read
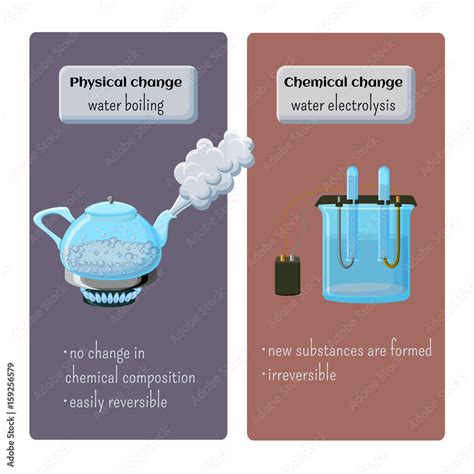
Table of Contents
Is Boiling Water a Physical Change or a Chemical Change? A Comprehensive Look
The question of whether boiling water represents a physical or chemical change is a fundamental one in chemistry, often encountered early in scientific education. While seemingly simple, a thorough understanding requires delving into the definitions of physical and chemical changes, examining the properties of water, and exploring the process of boiling at a molecular level. This article aims to provide a comprehensive answer, clarifying any misconceptions and highlighting the subtle yet crucial distinctions.
Understanding Physical and Chemical Changes
Before we dive into the specifics of boiling water, let's establish clear definitions of physical and chemical changes.
Physical Changes
A physical change alters the form or appearance of a substance but does not change its chemical composition. The fundamental building blocks of the substance – its molecules – remain the same. Examples include:
- Changes in state: Melting ice, boiling water, freezing liquid, sublimation (solid to gas), deposition (gas to solid). These changes involve a rearrangement of molecules but not a breaking or forming of chemical bonds.
- Changes in shape or size: Cutting paper, breaking a glass, bending a metal rod. The chemical makeup of the material remains unchanged.
- Dissolving: Salt dissolving in water is a physical change because the salt molecules are dispersed in the water but still retain their chemical identity. They can be recovered through evaporation.
Chemical Changes
A chemical change, also known as a chemical reaction, involves the formation of one or more new substances with different chemical properties. This happens when chemical bonds are broken and new bonds are formed, rearranging the atoms into different molecules. Examples include:
- Burning: Combustion reactions involve the rapid reaction of a substance with oxygen, producing heat and light, and forming new compounds like carbon dioxide and water.
- Rusting: The slow oxidation of iron in the presence of oxygen and water forms iron oxide, a completely different substance with different properties.
- Cooking: Many cooking processes involve chemical changes, such as the browning of meat (Maillard reaction) or the setting of an egg.
Analyzing the Boiling of Water
Now, let's apply these definitions to the process of boiling water. When water boils, it transitions from its liquid state to its gaseous state (steam). This is a phase transition, a type of physical change.
Molecular Perspective
At the molecular level, the water molecules (H₂O) in liquid water are relatively close together, held by weak intermolecular forces (hydrogen bonds). As heat is added, the kinetic energy of these molecules increases. This increased energy overcomes the intermolecular forces, allowing the molecules to escape the liquid phase and enter the gaseous phase as steam.
Crucially, the chemical bonds within each water molecule (the covalent bonds between hydrogen and oxygen atoms) remain intact throughout the boiling process. The water molecules are simply moving further apart and changing their state of aggregation, not undergoing any alteration in their chemical structure.
Observable Changes During Boiling
Several observable changes occur when water boils, further supporting the classification as a physical change:
- Change of state: The most obvious change is the transformation from liquid water to gaseous steam.
- Temperature remains constant: While heat is continuously added, the temperature of boiling water remains constant at 100°C (at standard atmospheric pressure). This constant temperature is characteristic of phase transitions and indicates that the energy is being used to break intermolecular forces rather than increase the kinetic energy of the molecules.
- Reversibility: The steam produced can be condensed back into liquid water through cooling, demonstrating the reversibility of the process. This reversibility is a hallmark of physical changes.
Addressing Common Misconceptions
Despite the clear evidence, some misconceptions can arise:
- Decomposition: Some might argue that boiling water leads to the decomposition of water into hydrogen and oxygen. This is incorrect. While water can be decomposed into its constituent elements through electrolysis (using electricity), boiling alone does not achieve this. The chemical bonds within the water molecules remain unbroken.
- Chemical reactions with dissolved substances: If impurities or dissolved substances are present in the water, they might undergo chemical changes during boiling. For example, certain minerals might precipitate out of solution. However, the boiling of the water itself remains a physical change. The changes to the dissolved substances are separate chemical processes.
Beyond Pure Water: Considering Impurities
The discussion so far has focused on pure water. However, most water we encounter contains dissolved minerals, gases, and other impurities. The boiling of such water can lead to some chemical changes concerning these impurities, but the boiling of the water molecule itself is still a physical change. For example:
- Dissolved gases escaping: Dissolved gases like carbon dioxide will escape from the water as it boils, but this is a physical change – the gas is simply being released from the solution.
- Mineral precipitation: Some dissolved minerals might precipitate out of solution as the water evaporates, forming scale. This is a chemical change affecting the minerals, not the water itself.
Conclusion: Boiling Water is a Physical Change
In conclusion, boiling water is unequivocally a physical change. While the state of the water changes from liquid to gas, the chemical composition of the water molecules remains unchanged. The process involves a change in the arrangement and kinetic energy of the water molecules, not the breaking and forming of chemical bonds. The key characteristics – constant boiling temperature, reversibility, and the absence of new chemical substances – all firmly place boiling water within the realm of physical changes. Although impurities might undergo separate chemical reactions during boiling, this does not alter the fundamental physical nature of the phase transition of the water itself. Understanding this distinction is crucial for grasping fundamental concepts in chemistry and appreciating the intricacies of matter.
Latest Posts
Latest Posts
-
1 4 Mile How Many Yards
Mar 16, 2025
-
Derivative Of X 2 1 3
Mar 16, 2025
-
What Is Equivalent To 2 5
Mar 16, 2025
-
The Quotient Of 45 And R
Mar 16, 2025
-
Square Root Of 137 Simplified Radical Form
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about Is Boiling Water A Physical Change Or Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
