Write The Numbers In Scientific Notation. 673.5
listenit
Mar 15, 2025 · 5 min read
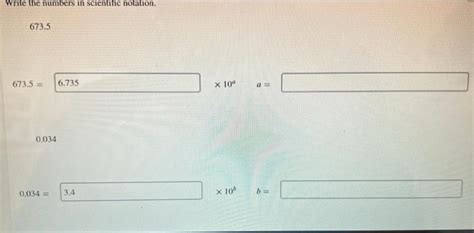
Table of Contents
Writing Numbers in Scientific Notation: A Comprehensive Guide
Scientific notation is a standardized way of writing very large or very small numbers. It simplifies the representation of these numbers, making them easier to read, write, and use in calculations. Understanding scientific notation is crucial in many fields, from science and engineering to finance and computer science. This comprehensive guide will delve into the intricacies of scientific notation, using the number 673.5 as a starting point and expanding to cover a wide range of applications and examples.
Understanding the Basics of Scientific Notation
Scientific notation expresses a number as a product of a coefficient and a power of 10. The coefficient is a number between 1 (inclusive) and 10 (exclusive), and the power of 10 indicates how many places the decimal point needs to be moved to obtain the original number. The general form is:
a x 10<sup>b</sup>
Where:
- a is the coefficient (1 ≤ a < 10)
- b is the exponent (an integer)
Converting 673.5 to Scientific Notation
Let's convert our example number, 673.5, into scientific notation.
-
Identify the coefficient: We need to move the decimal point to the left until we have a number between 1 and 10. In this case, we move the decimal point two places to the left, resulting in the coefficient 6.735.
-
Determine the exponent: Since we moved the decimal point two places to the left, the exponent is +2. Each place moved to the left adds 1 to the exponent.
-
Write the scientific notation: Combining the coefficient and the exponent, we get the scientific notation for 673.5 as:
6.735 x 10<sup>2</sup>
Working with Positive and Negative Exponents
The exponent in scientific notation indicates the magnitude of the number. A positive exponent indicates a large number (greater than 1), while a negative exponent indicates a small number (between 0 and 1).
Examples of Positive Exponents:
- 1,200,000: 1.2 x 10<sup>6</sup> (decimal moved 6 places to the left)
- 34,500: 3.45 x 10<sup>4</sup> (decimal moved 4 places to the left)
- 987,654,321: 9.87654321 x 10<sup>8</sup> (decimal moved 8 places to the left)
Examples of Negative Exponents:
- 0.00045: 4.5 x 10<sup>-4</sup> (decimal moved 4 places to the right)
- 0.00000078: 7.8 x 10<sup>-7</sup> (decimal moved 7 places to the right)
- 0.0000000000321: 3.21 x 10<sup>-11</sup> (decimal moved 11 places to the right)
Converting from Scientific Notation to Standard Form
The reverse process, converting from scientific notation back to standard form, is equally important. To do this, you move the decimal point according to the exponent. A positive exponent means moving the decimal point to the right, while a negative exponent means moving it to the left. Add zeros as needed to fill in the places.
Examples:
- 2.5 x 10<sup>3</sup>: Move the decimal point 3 places to the right, adding zeros as necessary, resulting in 2500.
- 7.89 x 10<sup>-2</sup>: Move the decimal point 2 places to the left, resulting in 0.0789.
- 1.01 x 10<sup>5</sup>: Move the decimal point 5 places to the right, resulting in 101000.
Performing Calculations with Scientific Notation
Scientific notation significantly simplifies calculations involving very large or very small numbers. When multiplying or dividing numbers in scientific notation, you multiply or divide the coefficients and add or subtract the exponents, respectively. When adding or subtracting, the exponents must be the same; if they are not, you must adjust one of the numbers to match the other's exponent.
Multiplication:
(a x 10<sup>b</sup>) x (c x 10<sup>d</sup>) = (a x c) x 10<sup>(b+d)</sup>
Division:
(a x 10<sup>b</sup>) / (c x 10<sup>d</sup>) = (a/c) x 10<sup>(b-d)</sup>
Addition and Subtraction:
To add or subtract numbers in scientific notation, both numbers must have the same exponent. If they don't, adjust one to match the other.
Example:
Add 2.5 x 10<sup>3</sup> and 4 x 10<sup>2</sup>.
- Adjust the exponents: Convert 4 x 10<sup>2</sup> to 0.4 x 10<sup>3</sup>.
- Add the coefficients: 2.5 + 0.4 = 2.9
- Result: 2.9 x 10<sup>3</sup>
Practical Applications of Scientific Notation
Scientific notation's widespread applications across various disciplines highlight its importance.
Science and Engineering:
- Astronomy: Representing vast distances (light-years) and sizes of celestial objects.
- Physics: Handling extremely small values (atomic particles, wavelengths).
- Chemistry: Expressing the number of atoms and molecules in chemical reactions (Avogadro's number).
- Computer Science: Measuring data storage capacity (bytes, gigabytes, terabytes).
Finance:
- National debts: Expressing enormous figures in a manageable format.
- Market capitalization: Representing the total value of companies' stocks.
Everyday Life:
While not explicitly used daily, the underlying principles are frequently applied in understanding large or small quantities, such as population figures, national budgets, or microscopic measurements.
Advanced Concepts and Considerations
Significant Figures and Scientific Notation:
When using scientific notation, it is crucial to maintain the correct number of significant figures to reflect the precision of the measurement. The coefficient should reflect the appropriate level of precision.
Rounding and Accuracy:
Rounding during calculations is necessary, and it’s important to choose the appropriate rounding method to minimize the impact on the overall accuracy.
Conclusion
Scientific notation is an invaluable tool for simplifying the representation and manipulation of extremely large and small numbers. Understanding its principles, from basic conversions to complex calculations, is crucial for proficiency in numerous fields. By mastering this system, you'll enhance your ability to process and communicate numerical data effectively, whether dealing with astronomical distances, microscopic measurements, or financial figures. The consistent application of the rules, attention to significant figures, and understanding of rounding techniques are key to accuracy and reliable results when working with scientific notation. This comprehensive guide aims to equip you with the necessary knowledge and skills to confidently navigate the world of scientific notation and harness its power to solve problems and express numerical data efficiently.
Latest Posts
Latest Posts
-
C 5 9 F 32 For F
Mar 15, 2025
-
What Is The Top Of A Wave Called
Mar 15, 2025
-
Log X 3 Log X 1
Mar 15, 2025
-
8x 4 4x 3 4 6x 4 4
Mar 15, 2025
-
What Do The Arrows On A Food Chain Represent
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Write The Numbers In Scientific Notation. 673.5 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
