Write The Formula Formula Unit For The Following Compounds
listenit
Apr 06, 2025 · 6 min read
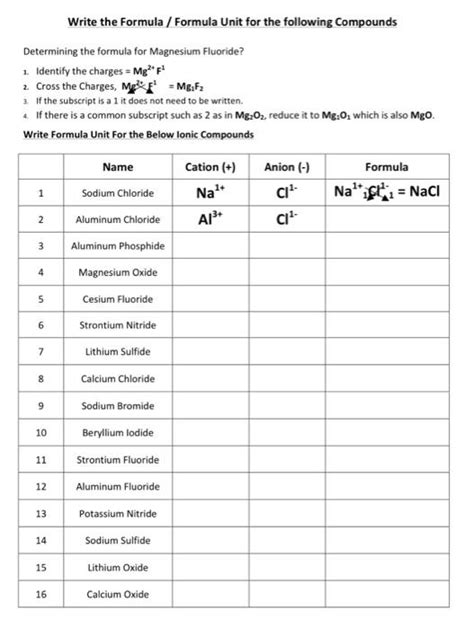
Table of Contents
Determining Formula Units for Chemical Compounds: A Comprehensive Guide
Understanding chemical formulas and how to derive them is fundamental to chemistry. This comprehensive guide delves into the process of determining the formula unit for various compounds, encompassing ionic compounds, covalent compounds, and hydrates. We'll explore the underlying principles, provide step-by-step examples, and offer tips for mastering this crucial skill.
Understanding Formula Units
A formula unit represents the simplest whole-number ratio of ions in an ionic compound or the simplest ratio of atoms in a molecule. It's the empirical formula, representing the smallest repeating unit in the crystal lattice structure for ionic compounds or the molecular structure for covalent compounds. Crucially, it's not necessarily the actual number of atoms or ions present in a single unit of the substance.
For ionic compounds, formula units are determined by balancing the charges of the constituent cations (positive ions) and anions (negative ions). The overall charge of the formula unit must be neutral (zero).
For covalent compounds, formula units represent the actual composition of a molecule. The subscripts in the formula indicate the number of each type of atom in a single molecule.
Determining Formula Units for Ionic Compounds
The process of determining the formula unit for an ionic compound involves several key steps:
-
Identify the cation and anion: Determine the individual ions that make up the compound. This usually requires knowledge of the charges of common ions. Remember that metals generally form positive ions (cations), while nonmetals usually form negative ions (anions). Transition metals can have multiple oxidation states, requiring careful consideration of the context.
-
Determine the charge of each ion: This is crucial for balancing the charges. Use a periodic table or a table of common ions as a reference. Remember to account for the possible different oxidation states of transition metals, which are often indicated in the compound name (e.g., iron(II) chloride vs. iron(III) chloride).
-
Balance the charges: To achieve a neutral formula unit, the total positive charge must equal the total negative charge. This often involves finding the least common multiple (LCM) of the charges to determine the appropriate subscripts for each ion.
-
Write the formula unit: The cation is written first, followed by the anion. The subscripts indicate the number of each ion needed to achieve charge neutrality. Subscripts of '1' are generally omitted.
Example 1: Sodium Chloride (NaCl)
- Cation: Sodium (Na⁺) - Charge: +1
- Anion: Chloride (Cl⁻) - Charge: -1
The charges are already balanced (+1 and -1), so the formula unit is simply NaCl.
Example 2: Magnesium Oxide (MgO)
- Cation: Magnesium (Mg²⁺) - Charge: +2
- Anion: Oxide (O²⁻) - Charge: -2
The charges are already balanced (+2 and -2), so the formula unit is MgO.
Example 3: Aluminum Oxide (Al₂O₃)
- Cation: Aluminum (Al³⁺) - Charge: +3
- Anion: Oxide (O²⁻) - Charge: -2
To balance the charges, we need two aluminum ions (+6 total charge) and three oxide ions (-6 total charge). Therefore, the formula unit is Al₂O₃. The LCM of 3 and 2 is 6.
Example 4: Iron(III) Sulfate (Fe₂(SO₄)₃)
- Cation: Iron(III) (Fe³⁺) - Charge: +3
- Anion: Sulfate (SO₄²⁻) - Charge: -2
The LCM of 3 and 2 is 6. To achieve a neutral charge, we need two iron(III) ions (+6) and three sulfate ions (-6). This leads to the formula unit Fe₂(SO₄)₃. Note the use of parentheses to indicate that the sulfate ion is a polyatomic ion.
Determining Formula Units for Covalent Compounds
Covalent compounds involve the sharing of electrons between atoms. Their formula units represent the actual number of atoms of each element in a molecule. Prefixes in the names of covalent compounds indicate the number of each type of atom.
- Mono- (1)
- Di- (2)
- Tri- (3)
- Tetra- (4)
- Penta- (5)
- Hexa- (6)
- Hepta- (7)
- Octa- (8)
- Nona- (9)
- Deca- (10)
**Example 5: Carbon Dioxide (CO₂) **
The name indicates one carbon atom and two oxygen atoms, resulting in the formula unit CO₂.
Example 6: Dinitrogen Tetroxide (N₂O₄)
The name indicates two nitrogen atoms and four oxygen atoms. The formula unit is N₂O₄.
Example 7: Phosphorus Pentachloride (PCl₅)
The name indicates one phosphorus atom and five chlorine atoms, resulting in the formula unit PCl₅.
Determining Formula Units for Hydrates
Hydrates are compounds that contain water molecules within their crystal structure. The water molecules are chemically bound but can often be removed through heating. The formula unit for a hydrate includes the formula unit of the anhydrous compound (the compound without water) and the number of water molecules associated with each formula unit.
The number of water molecules is indicated by a dot (·) followed by a numerical prefix (e.g., mono-, di-, tri-, etc.) or a numerical coefficient.
Example 8: Copper(II) Sulfate Pentahydrate (CuSO₄·5H₂O)
This compound consists of copper(II) sulfate (CuSO₄) and five water molecules (5H₂O) per formula unit. The formula unit is written as CuSO₄·5H₂O.
Example 9: Epsom Salt (MgSO₄·7H₂O)
Epsom salt is magnesium sulfate heptahydrate, indicating seven water molecules per formula unit of magnesium sulfate (MgSO₄). Its formula unit is MgSO₄·7H₂O.
Advanced Considerations and Challenges
While the process outlined above covers many common cases, certain situations require more advanced understanding:
-
Polyatomic Ions: Dealing with polyatomic ions (ions composed of multiple atoms) requires careful attention to both the charge and the overall composition of the ion. Parentheses are used to group polyatomic ions when more than one is present in the formula unit.
-
Transition Metal Ions: Transition metals can exhibit multiple oxidation states, leading to different charges. The oxidation state must be specified (often in the name of the compound) to determine the correct charge and thus the formula unit.
-
Complex Ions: Compounds containing complex ions (ions with a central metal atom surrounded by ligands) require a more in-depth understanding of coordination chemistry.
-
Empirical vs. Molecular Formulas: While formula units often represent empirical formulas (simplest whole-number ratios), for covalent compounds, the formula unit may also be the molecular formula, representing the actual number of atoms in a molecule.
Mastering Formula Unit Determination: Tips and Practice
Proficiency in determining formula units comes with practice. Here are some helpful tips:
-
Master the charges of common ions: Create flashcards or use other memorization techniques to learn the charges of common cations and anions.
-
Understand the concept of charge balance: The overall charge of a formula unit must always be zero.
-
Practice, practice, practice: Work through numerous examples of varying complexity. Start with simple ionic compounds and gradually progress to more challenging ones involving polyatomic ions, transition metals, and hydrates.
-
Utilize online resources: Numerous online resources, including interactive tutorials and quizzes, can help you reinforce your understanding.
-
Seek help when needed: Don't hesitate to ask your teacher, professor, or tutor for assistance if you encounter difficulties.
By systematically following these steps and practicing regularly, you can confidently determine the formula units of a wide range of chemical compounds. This fundamental skill is crucial for further progress in chemistry and related fields. Remember that understanding the underlying principles of charge balance and stoichiometry is key to success.
Latest Posts
Latest Posts
-
1 3 Divided By 4 Fraction
Apr 08, 2025
-
How To Solve Quartic Equation In Calculator
Apr 08, 2025
-
What Is 35 Percent Of 60
Apr 08, 2025
-
Whats The Square Root Of 36
Apr 08, 2025
-
120 Is What Percent Of 180
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about Write The Formula Formula Unit For The Following Compounds . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.