Why Does The Atomic Radii Increase Down A Group
listenit
Mar 15, 2025 · 6 min read
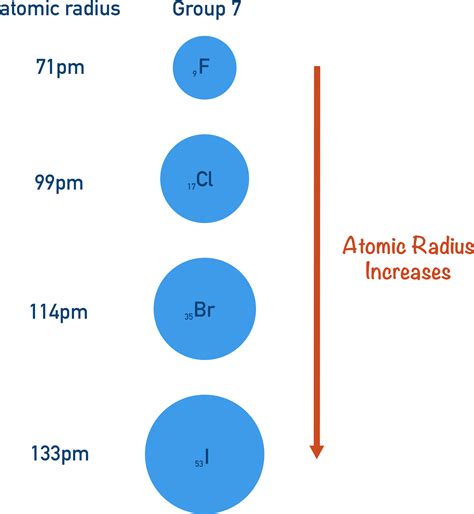
Table of Contents
Why Does Atomic Radius Increase Down a Group? A Deep Dive into Periodic Trends
Understanding the periodic trends of elements is fundamental to grasping the principles of chemistry. One of the most important and readily observable trends is the increase in atomic radius as you move down a group (or column) in the periodic table. This seemingly simple observation, however, stems from a complex interplay of fundamental physical forces and quantum mechanical effects. This article will delve into the detailed explanation of why atomic radius increases down a group, exploring the underlying principles and addressing common misconceptions.
The Definition of Atomic Radius
Before we explore why atomic radius increases, let's define what we mean by atomic radius. Atomic radius isn't a precisely measurable quantity like the diameter of a marble. Instead, it's a measure of the size of an atom, typically defined as half the distance between the nuclei of two identical atoms bonded together. This definition is used for covalent radii. For metallic radii, it's half the distance between adjacent metal atoms in a metallic lattice. Understanding this distinction is crucial, as the method of measurement can subtly influence the precise value obtained. However, the overall trend—increase down a group—remains consistent regardless of the specific definition used.
The Key Player: Electron Shells
The primary reason for the increase in atomic radius down a group is the addition of electron shells. As we move down a group, each successive element adds an electron to a new, higher energy level principal quantum number (n). This new electron shell is located further away from the nucleus. Think of it like adding layers to an onion: each layer increases the overall size. Each new shell corresponds to a significant increase in distance from the nucleus, directly influencing the atomic radius.
Principal Quantum Number (n) and its Influence
The principal quantum number (n) determines the energy level of an electron and, consequently, its average distance from the nucleus. As you descend a group, 'n' increases, meaning electrons occupy orbitals that are progressively further from the nucleus. This increased distance is the core reason for the expansion of atomic size. For example, the valence electrons of lithium (Li) are in the n=2 shell, whereas those of sodium (Na) are in the n=3 shell, and those of potassium (K) are in the n=4 shell. This progressively larger 'n' value directly translates to a larger atomic radius.
Shielding Effect: Inner Electrons' Protective Role
While the addition of electron shells is the primary driver, the shielding effect plays a significant supporting role. Inner electrons (those in lower energy levels) partially shield the outer electrons from the positive charge of the nucleus. This shielding effect reduces the effective nuclear charge experienced by the valence electrons. The more inner electrons present, the greater the shielding effect.
Effective Nuclear Charge (Z<sub>eff</sub>)
The effective nuclear charge (Z<sub>eff</sub>) is the net positive charge experienced by an electron after accounting for the shielding effect of other electrons. It's calculated as the difference between the actual nuclear charge (Z) and the shielding constant (S): Z<sub>eff</sub> = Z - S. While the nuclear charge increases down a group, the increase in shielding from the added inner electrons is more significant. This results in a relatively smaller increase in Z<sub>eff</sub> compared to the increase in the distance of the valence electrons from the nucleus. The weaker attraction between the nucleus and the outer electrons contributes to the larger atomic radius.
The Role of Electron-Electron Repulsion
Another factor contributing to the increase in atomic radius is electron-electron repulsion. As the number of electrons increases down a group, the repulsive forces between these electrons also increase. This repulsion pushes the outer electrons further away from the nucleus, further enlarging the atom. This effect, while less dominant than the addition of electron shells and shielding, adds to the overall trend of increasing atomic radius.
Comparing Across Periods: A Contrast
It's important to contrast the trend down a group with the trend across a period (or row). Across a period, the atomic radius generally decreases. This is because, while the number of electrons increases, the additional electrons are added to the same principal energy level. The increase in nuclear charge outweighs the effect of electron-electron repulsion, resulting in a stronger attraction between the nucleus and the electrons, thus causing a decrease in the atomic radius.
Exceptions and Nuances: Transition Metals and Lanthanides/Actinides
While the general trend of increasing atomic radius down a group holds true, there can be minor exceptions and nuances, particularly with transition metals and the lanthanides/actinides. The poor shielding effect of d and f electrons can cause slight irregularities. The lanthanide contraction, for instance, refers to the unexpectedly small size of the lanthanide elements. This is due to the poor shielding effect of 4f electrons, resulting in a stronger effective nuclear charge and a smaller than expected atomic radius. However, even with these exceptions, the overall trend of increasing atomic radius down a group remains valid.
Importance of Understanding Atomic Radius
Understanding the trend of increasing atomic radius down a group is crucial for several reasons:
-
Predicting Chemical Properties: Atomic radius influences an element's reactivity and bonding behavior. Larger atoms generally have lower ionization energies (easier to remove electrons) and lower electronegativities (less attraction for electrons), influencing the types of chemical bonds they form.
-
Interpreting Periodic Trends: The atomic radius is interconnected with other periodic trends like ionization energy, electronegativity, and electron affinity. Understanding one helps to understand the others.
-
Material Science and Engineering: Atomic radius is a critical factor in materials science, determining the properties of solids, such as density, conductivity, and mechanical strength. The size of atoms directly affects how they pack together in a solid lattice.
-
Nuclear Chemistry: Atomic radius has implications in nuclear chemistry, particularly in determining the stability and reactivity of isotopes.
Conclusion: A Holistic Perspective
The increase in atomic radius down a group is a consequence of the combined effects of several factors working together. The dominant force is the addition of electron shells to higher principal quantum numbers (n). This is further augmented by the shielding effect of inner electrons and the increasing electron-electron repulsion. While minor exceptions and irregularities can occur, especially with transition metals and inner transition elements, the overall trend remains robust and constitutes a fundamental principle in chemistry. Understanding this trend provides a valuable tool for predicting and explaining the chemical and physical properties of elements and their compounds. Furthermore, a deep understanding of atomic radius lays a strong foundation for exploring more advanced concepts in chemistry and related fields.
Latest Posts
Latest Posts
-
C 5 9 F 32 For F
Mar 15, 2025
-
What Is The Top Of A Wave Called
Mar 15, 2025
-
Log X 3 Log X 1
Mar 15, 2025
-
8x 4 4x 3 4 6x 4 4
Mar 15, 2025
-
What Do The Arrows On A Food Chain Represent
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Why Does The Atomic Radii Increase Down A Group . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
