Which State Of Matter Has The Most Kinetic Energy
listenit
Mar 19, 2025 · 5 min read
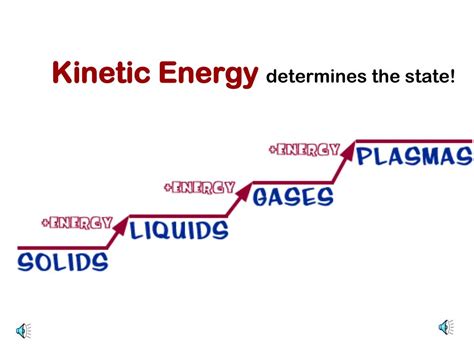
Table of Contents
Which State of Matter Has the Most Kinetic Energy?
The question of which state of matter possesses the most kinetic energy isn't as straightforward as it might initially seem. While the simplistic answer often points towards gases, a deeper dive reveals a more nuanced understanding involving factors like temperature, specific heat capacity, and the very definition of "kinetic energy" in different states. This article explores the complexities of kinetic energy within solids, liquids, and gases, aiming to provide a comprehensive and insightful answer.
Understanding Kinetic Energy and States of Matter
Before delving into the specifics of each state, let's establish a clear understanding of the fundamental concepts:
Kinetic Energy: This is the energy an object possesses due to its motion. At the molecular level, it relates to the constant vibrational, rotational, and translational movement of atoms and molecules. The faster these particles move, the higher their kinetic energy.
States of Matter: The three primary states—solid, liquid, and gas—differ fundamentally in how their constituent particles interact and move.
-
Solids: Particles are tightly packed in a rigid structure, exhibiting minimal translational movement. Their kinetic energy is primarily manifested as vibrational motion around fixed points.
-
Liquids: Particles are closer together than in gases but have more freedom of movement than in solids. They exhibit both vibrational and translational motion, with kinetic energy distributed between both.
-
Gases: Particles are widely dispersed, moving freely and independently. Their kinetic energy is predominantly translational, with significant random motion in all directions.
Kinetic Energy in Solids
While solids appear static, their particles are constantly vibrating within their lattice structure. The amplitude of these vibrations increases with temperature. However, the kinetic energy in solids remains relatively low compared to liquids and gases due to the strong intermolecular forces holding the particles in place. The restricted movement limits the overall kinetic energy. Think of it like a tightly packed crowd – individuals can vibrate and jostle, but their overall movement is constrained.
Factors Affecting Kinetic Energy in Solids:
- Temperature: Higher temperatures lead to greater vibrational amplitudes and thus, higher kinetic energy.
- Bond Strength: Stronger intermolecular forces restrict particle movement, leading to lower kinetic energy at a given temperature.
- Material Properties: Different materials have different vibrational frequencies and capacities, influencing their kinetic energy content.
Kinetic Energy in Liquids
Liquids represent an intermediate state between solids and gases. Their particles have more freedom of movement than solids but are still relatively close together. This allows for both vibrational and translational motion, leading to a higher average kinetic energy than solids at the same temperature. The particles can slide past each other, leading to fluidity, but the intermolecular forces are still significant enough to maintain a defined volume.
Factors Affecting Kinetic Energy in Liquids:
- Temperature: Similar to solids, increased temperature translates to increased kinetic energy, resulting in faster particle movement and potentially a decrease in viscosity.
- Intermolecular Forces: Weaker intermolecular forces allow for greater particle mobility and hence higher kinetic energy at a given temperature compared to solids with stronger forces.
- Density: Higher density liquids generally possess lower kinetic energy at the same temperature due to increased interparticle interactions.
Kinetic Energy in Gases
Gases exhibit the highest kinetic energy among the three primary states of matter. Their particles are widely separated and move randomly with high translational velocities. The weak intermolecular forces allow for almost completely free movement, resulting in a much higher average kinetic energy than solids or liquids at the same temperature. The kinetic energy is almost entirely translational, as opposed to solids and liquids with a significant vibrational component.
Factors Affecting Kinetic Energy in Gases:
- Temperature: A direct and significant relationship exists between temperature and kinetic energy in gases. Higher temperatures lead to significantly higher kinetic energy.
- Pressure: Increased pressure forces gas particles closer together, increasing the frequency of collisions and potentially slightly increasing average kinetic energy (though temperature remains the dominant factor).
- Molecular Weight: Lighter gas molecules move faster at the same temperature, possessing higher average kinetic energy than heavier molecules.
The Nuance of the Answer
While gases generally possess the highest kinetic energy at a given temperature, it's crucial to acknowledge the complexities:
- Temperature Dependence: At very low temperatures, the differences in kinetic energy between the states might become less pronounced. Near absolute zero, the kinetic energy of all states approaches zero.
- Specific Heat Capacity: Different substances have different specific heat capacities. This means that the same amount of heat energy will cause a different temperature change and thus a different change in kinetic energy for different substances, regardless of their state.
- Phase Transitions: The energy involved in phase transitions (melting, boiling) significantly impacts the overall energy of a system. The energy required to overcome intermolecular forces during phase transitions adds considerable energy, affecting the final kinetic energy balance.
Conclusion: Context is Key
The simplest answer is that gases generally have the highest kinetic energy at a given temperature. However, this statement is conditional and depends on the interplay of temperature, pressure, intermolecular forces, and specific heat capacities of the materials involved. A detailed comparison requires a more nuanced understanding of these factors and the conditions under which the comparison is made. While gases demonstrate the highest average translational kinetic energy, the overall energy picture is more complex and requires considering all factors involved in determining the system's total kinetic energy. It's not a simple comparison, but rather a complex interplay of multiple factors.
Latest Posts
Latest Posts
-
Greatest Common Factor Of 16 And 18
Mar 20, 2025
-
Do The Quotations Go After The Period
Mar 20, 2025
-
What Type Of Organism Is Grass
Mar 20, 2025
-
What Is 8 To The Power Of 2
Mar 20, 2025
-
Which Of These Is A Regulatory Gene
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Which State Of Matter Has The Most Kinetic Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
