Which State Of Matter Has A Definite Shape And Volume
listenit
Mar 22, 2025 · 5 min read
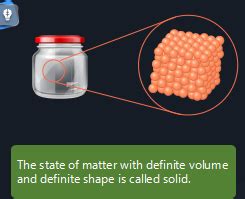
Table of Contents
Which State of Matter Has a Definite Shape and Volume? Understanding Solids
The question of which state of matter possesses a definite shape and volume boils down to understanding the fundamental properties of matter at a molecular level. While gases and liquids are known for their fluidity and adaptability, solids stand out as the only state of matter that consistently exhibits both a definite shape and a definite volume. This characteristic stems from the unique arrangement and interactions of their constituent particles.
The Defining Characteristics of Solids
Let's delve deeper into the defining properties of solids that lead to their fixed shape and volume:
Strong Intermolecular Forces
Unlike gases and liquids where molecules are relatively free to move around, the molecules in a solid are held together by strong intermolecular forces. These forces can be covalent bonds (as seen in diamond), ionic bonds (like in table salt), metallic bonds (in metals), or strong intermolecular forces like hydrogen bonds (in ice). These strong attractions restrict the movement of particles, keeping them in close proximity and in a fixed arrangement.
Regular and Ordered Arrangement
This strong bonding results in a regular and ordered arrangement of particles within a solid. This arrangement, often referred to as a crystal lattice, is a repeating three-dimensional pattern that determines the macroscopic shape and properties of the solid. While some solids may be amorphous (lacking a long-range ordered structure), even these exhibit limited molecular movement and maintain a fixed shape and volume.
Minimal Kinetic Energy
The particles in a solid possess minimal kinetic energy, meaning they vibrate in place rather than moving freely. This limited movement prevents the solid from changing its shape or volume unless acted upon by external forces such as pressure or temperature changes that can overcome the intermolecular forces.
Incompressibility
The close packing of particles in a solid results in incompressibility. Unlike gases, which are easily compressed due to the large spaces between molecules, applying pressure to a solid has little effect on its volume because there is very little space between the tightly packed particles.
Examples of Solids with Definite Shape and Volume
The everyday world is full of examples illustrating this key characteristic of solids:
- Ice cubes: Maintain their cubic shape and volume until they melt.
- Rocks: Retain their form and size unless physically broken or eroded.
- Metals: Whether cast into intricate shapes or forged into tools, metallic solids maintain their form.
- Crystals: Demonstrate the ordered structure beautifully through their geometric shapes and consistent volume.
- Wood: While its structure is more complex than simple crystals, wood still retains its shape and volume, barring external forces like burning or decay.
Contrast with Liquids and Gases
Understanding why solids have a definite shape and volume requires comparing them to liquids and gases:
Liquids: Definite Volume, Indefinite Shape
Liquids possess a definite volume but an indefinite shape. Their molecules are closer together than in gases but still have enough kinetic energy to move past each other. This allows liquids to conform to the shape of their container while maintaining a constant volume. The intermolecular forces in liquids are weaker than in solids, allowing for greater molecular movement and fluidity.
Gases: Indefinite Shape and Volume
Gases have both an indefinite shape and an indefinite volume. The intermolecular forces in gases are extremely weak, allowing molecules to move freely and independently. Gases expand to fill whatever container they occupy, adopting both the shape and volume of their surroundings.
Exceptions and Considerations
While the rule holds true for the vast majority of solids, some exceptions and nuances exist:
Amorphous Solids
Amorphous solids, such as glass and rubber, lack the long-range ordered structure of crystalline solids. Their molecules are arranged randomly, resulting in a less rigid structure. However, even amorphous solids maintain a definite shape and volume, though they might exhibit some degree of elasticity or plasticity.
Temperature and Pressure Effects
Extreme changes in temperature or pressure can influence the shape and volume of solids. High temperatures can overcome the intermolecular forces, causing melting and a transition to the liquid state. Similarly, immense pressure can compress some solids to a degree, although the change is usually minimal compared to the compression of gases.
Sublimation
Certain solids can undergo sublimation, transitioning directly from the solid to the gaseous state without passing through the liquid phase. Dry ice (solid carbon dioxide) is a classic example. While undergoing sublimation, the solid loses its definite shape and volume, transitioning to the indefinite shape and volume of a gas.
The Importance of Understanding States of Matter
Understanding the different states of matter and their defining characteristics is fundamental to various scientific disciplines, including:
- Chemistry: Exploring chemical reactions and properties of substances in different phases.
- Physics: Investigating the behavior of matter under various conditions of temperature and pressure.
- Materials Science: Developing new materials with specific properties based on their molecular arrangement and interactions.
- Geology: Understanding the formation and behavior of rocks and minerals.
- Meteorology: Predicting weather patterns based on the behavior of water in its various states.
Conclusion: Solids – The Defined State
In conclusion, solids are the only state of matter that consistently exhibits both a definite shape and a definite volume. This defining property stems from the strong intermolecular forces holding their particles in a fixed, ordered arrangement with minimal kinetic energy. While exceptions and nuances exist, particularly with amorphous solids and under extreme conditions, the fundamental principle remains consistent: the tightly packed and strongly bonded nature of solid molecules ensures they maintain their form and volume unless acted upon by significant external forces. This understanding is crucial for comprehending the fundamental properties of matter and its behavior across various scientific fields.
Latest Posts
Latest Posts
-
Which Enzyme Unzips The Dna Double Helix
May 09, 2025
-
Which Element Is The Least Electronegative
May 09, 2025
-
Which Ratio Is Equivalent To 7 3
May 09, 2025
-
What Is The Basic Unit For Protein
May 09, 2025
-
How Many Gallons In 4 5 Liters
May 09, 2025
Related Post
Thank you for visiting our website which covers about Which State Of Matter Has A Definite Shape And Volume . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.