Which Of The Following Is Stronger Acid
listenit
Mar 25, 2025 · 5 min read
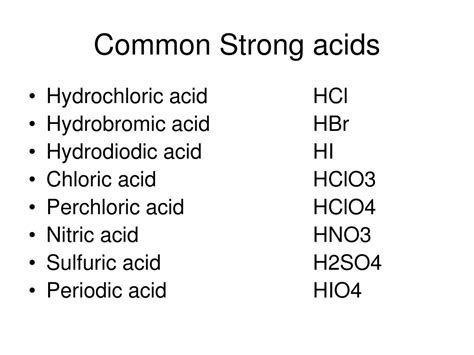
Table of Contents
Which is the Stronger Acid? A Deep Dive into Acid Strength
Determining which of two acids is stronger involves understanding the factors that influence acidity. While a simple glance at a table of pKa values can offer a quick answer, a deeper understanding of the underlying chemistry provides a more robust and insightful approach. This article will explore various aspects of acid strength, providing a comprehensive guide to comparing acids and predicting their relative strengths.
Understanding Acid Strength: The Basics
An acid, according to the Brønsted-Lowry definition, is a substance that donates a proton (H⁺). The strength of an acid is determined by its tendency to donate this proton. A strong acid readily donates its proton, resulting in almost complete dissociation in water. Conversely, a weak acid only partially dissociates, meaning a significant portion remains in its undissociated form in an aqueous solution.
This tendency to donate a proton is quantified by the acid dissociation constant (Ka). Ka is the equilibrium constant for the dissociation of an acid in water:
HA(aq) + H₂O(l) ⇌ H₃O⁺(aq) + A⁻(aq)
Ka = [H₃O⁺][A⁻] / [HA]
A larger Ka value indicates a stronger acid, signifying a greater extent of dissociation. For convenience, the pKa value (pKa = -log₁₀Ka) is often used. A smaller pKa value corresponds to a stronger acid.
Factors Affecting Acid Strength
Several key factors influence the strength of an acid:
1. Electronegativity of the Atom Bonded to Hydrogen
The more electronegative the atom bonded to the hydrogen atom, the more readily the proton is donated. This is because the electronegative atom pulls the electron density away from the hydrogen atom, weakening the H-X bond and facilitating proton donation. For example, hydrofluoric acid (HF) is a weaker acid than hydrochloric acid (HCl) because fluorine is more electronegative than chlorine. While fluorine is more electronegative, the stronger H-F bond offsets this effect making it weaker.
2. Bond Strength
A weaker H-X bond leads to a stronger acid. The easier it is to break the bond and release the proton, the stronger the acid. This is related to the size of the atom bonded to hydrogen. Larger atoms have longer, weaker bonds, making them better at donating protons. This explains why hydroiodic acid (HI) is a stronger acid than hydrobromic acid (HBr), which is stronger than hydrochloric acid (HCl).
3. Resonance Stabilization of the Conjugate Base
After an acid donates a proton, it forms its conjugate base. If the conjugate base is stabilized by resonance, it increases the acidity of the original acid. Resonance delocalizes the negative charge over multiple atoms, making the conjugate base more stable and therefore favoring the dissociation of the acid. Carboxylic acids, for example, are relatively strong acids due to the resonance stabilization of their carboxylate conjugate bases.
4. Inductive Effects
Electron-withdrawing groups (EWGs) attached to the acid molecule can increase acidity by pulling electron density away from the O-H bond, weakening it and making proton donation easier. Conversely, electron-donating groups (EDGs) decrease acidity. The strength of the inductive effect depends on the distance from the EWG or EDG to the acidic proton; the closer the group, the stronger the effect.
5. Hybridization of the Atom Bonded to Hydrogen
The hybridization of the atom bonded to hydrogen also influences acidity. The greater the s-character of the hybrid orbital, the more electronegative the atom will be, making the acid stronger. For instance, sp-hybridized carbon is more electronegative than sp²-hybridized carbon, resulting in stronger acidity for acetylene compared to ethylene.
6. Solvent Effects
The solvent in which the acid is dissolved can significantly influence its apparent strength. Water is a common solvent, but other solvents with different polarities can affect the extent of acid dissociation. Protic solvents (solvents with O-H or N-H bonds) can stabilize both the acid and its conjugate base through hydrogen bonding, thereby affecting the equilibrium.
Comparing Specific Acids: Examples
Let's consider a few examples to illustrate how these factors influence acid strength comparisons:
Example 1: Hydrochloric acid (HCl) vs. Acetic acid (CH₃COOH)
HCl is a strong acid (pKa ≈ -7) while acetic acid is a weak acid (pKa ≈ 4.76). The difference arises primarily from bond strength and the stability of the conjugate base. The H-Cl bond is much weaker than the O-H bond in acetic acid. Additionally, the chloride ion (Cl⁻) is a much weaker base than the acetate ion (CH₃COO⁻). The acetate ion is stabilized by resonance, but this stabilization is not enough to overcome the stronger O-H bond and the greater basicity of the acetate ion.
Example 2: Trichloroacetic acid (CCl₃COOH) vs. Acetic acid (CH₃COOH)
Trichloroacetic acid is a stronger acid than acetic acid. This is due to the inductive effect of the three chlorine atoms. Chlorine is an electron-withdrawing group, pulling electron density away from the O-H bond in trichloroacetic acid, weakening it and facilitating proton donation. This effect is stronger than the resonance stabilization in the acetate ion.
Example 3: Phosphoric acid (H₃PO₄) vs. Hypophosphorous acid (H₃PO₂)
Phosphoric acid has three acidic protons, while hypophosphorous acid has only one. The acidity of each proton varies, but generally, phosphoric acid is considered a weaker acid than hypophosphorous acid. This is due to a combination of factors, including the number of oxygen atoms directly bonded to the phosphorus and inductive effects within the molecules.
Advanced Considerations: Beyond pKa Values
While pKa values provide a convenient way to compare acid strengths, they are not always sufficient, especially when considering complex molecules or unusual solvents. In such cases, a more detailed analysis of the factors mentioned above is necessary. Quantum chemical calculations can also provide accurate predictions of acid strengths for molecules where experimental data is unavailable or difficult to obtain. Furthermore, understanding the context of the application is crucial. The apparent strength of an acid can depend significantly on the reaction conditions, such as concentration and temperature.
Conclusion: A Multifaceted Property
Acid strength is a multifaceted property influenced by several interacting factors. While pKa values provide a useful guide, a thorough understanding of the underlying chemistry, including electronegativity, bond strength, resonance, inductive effects, and solvent effects, is necessary for accurate predictions and insightful comparisons. This knowledge is crucial not only for academic pursuits but also for various applications in chemistry, biology, and other fields. By combining experimental data with theoretical understanding, a comprehensive assessment of acid strength can be achieved, leading to a deeper appreciation of this fundamental chemical property.
Latest Posts
Latest Posts
-
How Many Ounces Is 400 Mg
Mar 28, 2025
-
What Percent Is 40 Out Of 60
Mar 28, 2025
-
What 3 Subatomic Particles Make Up An Atom
Mar 28, 2025
-
What Is 3 6 As A Fraction
Mar 28, 2025
-
How Many Cups In 1 2 Gal
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Is Stronger Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
