Which Of The Following Has The Largest Atomic Radius
listenit
Mar 19, 2025 · 5 min read
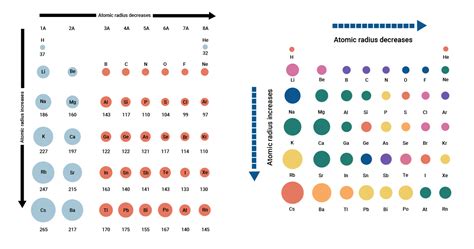
Table of Contents
Which of the Following Has the Largest Atomic Radius? Understanding Atomic Size Trends
Determining which atom possesses the largest atomic radius from a given set requires a solid understanding of periodic trends. Atomic radius, the distance from the atom's nucleus to its outermost electron shell, isn't a fixed value; it varies systematically across the periodic table. This article delves into the factors influencing atomic size, explains the trends, and provides a framework for comparing atomic radii effectively. We'll cover the key concepts and then apply them to solve a hypothetical example.
Factors Affecting Atomic Radius
Several factors interplay to determine an atom's size:
1. Principal Quantum Number (n):
This number represents the energy level of an electron. Higher energy levels (larger 'n' values) correspond to larger orbitals, pushing electrons further from the nucleus. Therefore, atoms with higher principal quantum numbers generally have larger atomic radii.
2. Effective Nuclear Charge (Z<sub>eff</sub>):
This is the net positive charge experienced by an electron in a multi-electron atom. It's less than the actual nuclear charge (Z) because inner electrons shield outer electrons from the full attractive force of the nucleus. A higher effective nuclear charge pulls outer electrons closer, resulting in a smaller atomic radius.
3. Shielding Effect:
Inner electrons shield outer electrons from the full positive charge of the nucleus. The more inner electrons present, the greater the shielding effect, reducing the effective nuclear charge felt by outer electrons. This leads to a larger atomic radius.
4. Electron-Electron Repulsion:
As the number of electrons increases, the repulsive forces between them also increase. This repulsion counteracts the attractive force of the nucleus, expanding the electron cloud and increasing the atomic radius.
Periodic Trends in Atomic Radius
Understanding the periodic trends in atomic radius is crucial for comparing the sizes of different atoms.
1. Across a Period (Left to Right):
As you move across a period from left to right, the atomic radius generally decreases. While the principal quantum number remains constant, the nuclear charge increases. This increased positive charge pulls the electrons closer, despite the added electron-electron repulsion. The increase in effective nuclear charge outweighs the increase in electron-electron repulsion.
2. Down a Group (Top to Bottom):
As you move down a group from top to bottom, the atomic radius generally increases. This is primarily due to the addition of electron shells (higher principal quantum number). The increased distance of the outermost electrons from the nucleus significantly outweighs the increase in nuclear charge.
Comparing Atomic Radii: A Step-by-Step Approach
To compare the atomic radii of different elements, follow these steps:
-
Locate the elements on the periodic table: Identify their periods and groups.
-
Consider the period trend: If the elements are within the same period, the element furthest to the left will have the largest atomic radius.
-
Consider the group trend: If the elements are within the same group, the element furthest down will have the largest atomic radius.
-
Combine trends: If the elements are in different periods and groups, consider both trends simultaneously. The element with the lowest effective nuclear charge and highest principal quantum number will typically have the largest radius.
-
Consider exceptions: While these trends are generally reliable, there can be minor exceptions due to specific electron configurations or other subtle factors.
Hypothetical Example: Comparing Atomic Radii
Let's consider a hypothetical example: Which of the following elements has the largest atomic radius: Lithium (Li), Sodium (Na), Fluorine (F), and Oxygen (O)?
-
Location on the periodic table: Li and Na are in Group 1 (alkali metals), while F and O are in Period 2.
-
Period trend: Within Period 2, Li has a larger atomic radius than F and O. This is because Li is further to the left.
-
Group trend: Within Group 1, Na has a larger atomic radius than Li because it is lower in the group.
-
Combining trends: Comparing all four elements, Na has the largest atomic radius. It is placed lower in its group and thus possesses a higher principal quantum number and a larger electron cloud compared to the others. It experiences a lower effective nuclear charge than the period 2 elements despite having a higher nuclear charge.
Therefore, in this example, Sodium (Na) possesses the largest atomic radius.
Advanced Considerations: Isoelectronic Series and Anions/Cations
The principles discussed above primarily apply to neutral atoms. However, let's extend our understanding to include isoelectronic species and ions.
Isoelectronic Series:
Isoelectronic species are atoms or ions that have the same number of electrons. For example, O²⁻, F⁻, Ne, Na⁺, and Mg²⁺ all have 10 electrons. Within an isoelectronic series, the atomic radius decreases as the nuclear charge increases. Therefore, Mg²⁺ would have the smallest radius, and O²⁻ the largest, in this series. The increased positive charge of the nucleus strongly attracts the electrons, leading to a smaller radius.
Anions and Cations:
Anions (negatively charged ions) have a larger atomic radius than their corresponding neutral atoms. The addition of electrons increases electron-electron repulsion and expands the electron cloud. Conversely, cations (positively charged ions) have a smaller atomic radius than their corresponding neutral atoms. The removal of electrons reduces electron-electron repulsion and allows the nucleus to pull the remaining electrons closer.
Conclusion: Mastering Atomic Radius Comparisons
Understanding atomic radius trends is fundamental to comprehending the behavior of elements and their compounds. By applying the principles outlined above – considering principal quantum number, effective nuclear charge, shielding effect, and electron-electron repulsion – we can reliably compare the atomic radii of different atoms and ions. Remember that while general trends exist, nuanced exceptions can arise due to electron configurations and other subtle interactions. Through systematic analysis, one can confidently determine which element exhibits the largest atomic radius from a given set. This knowledge is vital in various aspects of chemistry, including predicting chemical properties and reactivity. Continuing to explore these concepts and applying them to diverse examples will solidify your understanding and allow you to navigate the complexities of atomic structure with greater ease.
Latest Posts
Latest Posts
-
Can A Standard Deviation Be 0
Mar 19, 2025
-
How Many Protons Are In Au
Mar 19, 2025
-
What Are The Units Of Potential Energy
Mar 19, 2025
-
How Many Gallons In 30 Liters
Mar 19, 2025
-
4 2n 3 5n 3 2
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Has The Largest Atomic Radius . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
