Which Is The Element With The Lowest Electronegativity
listenit
Apr 07, 2025 · 5 min read
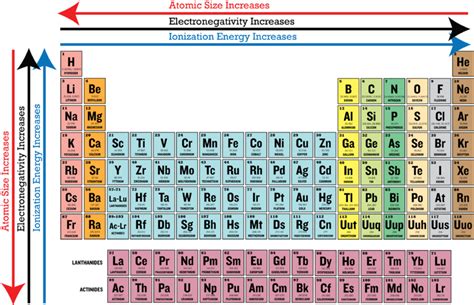
Table of Contents
Which Element Possesses the Lowest Electronegativity? Understanding Electronegativity and its Trends
Electronegativity, a fundamental concept in chemistry, dictates an atom's ability to attract electrons within a chemical bond. Understanding electronegativity is crucial for predicting the type of bond formed between atoms (ionic, covalent, or polar covalent) and understanding the behavior of molecules. While many elements exhibit varying degrees of electronegativity, a clear winner emerges when considering the element with the lowest electronegativity: francium.
Understanding Electronegativity: A Deep Dive
Before we delve into the specifics of francium, let's establish a solid foundation on electronegativity itself. Electronegativity isn't a directly measurable quantity like mass or charge. Instead, it's a relative property, representing the tendency of an atom to attract bonding electrons towards itself when it's part of a molecule. Several scales exist to quantify electronegativity, the most widely used being the Pauling scale.
Factors Influencing Electronegativity:
Several factors contribute to an atom's electronegativity:
- Nuclear Charge: A higher nuclear charge (more protons) exerts a stronger pull on electrons, increasing electronegativity.
- Atomic Radius: A larger atomic radius implies electrons are further from the nucleus, experiencing weaker attraction and resulting in lower electronegativity.
- Shielding Effect: Inner electrons shield outer electrons from the full positive charge of the nucleus. Increased shielding reduces the effective nuclear charge felt by valence electrons, lowering electronegativity.
- Electron Configuration: Atoms with nearly complete valence shells (e.g., halogens) have a strong tendency to gain electrons, exhibiting high electronegativity. Conversely, atoms with few valence electrons readily lose them, displaying low electronegativity.
The Champion of Low Electronegativity: Francium (Fr)
Francium, a radioactive alkali metal, proudly claims the title of the element with the lowest electronegativity. Its position in the periodic table, at the bottom left corner, perfectly explains this characteristic. Let's break down why:
- Extremely Large Atomic Radius: Francium possesses the largest atomic radius among all known elements. This significant distance between the nucleus and the outermost electrons dramatically weakens the attractive force, leading to its exceptionally low electronegativity.
- Weak Effective Nuclear Charge: Due to its large atomic radius and the shielding effect of numerous inner electrons, the effective nuclear charge experienced by the valence electron in francium is minimal. This significantly reduces its ability to attract electrons in a chemical bond.
- Low Ionization Energy: Francium readily loses its single valence electron to achieve a stable electron configuration, further indicating its low electronegativity. This ease of electron loss is a hallmark of alkali metals.
Comparing Francium to Other Low Electronegativity Elements:
While francium holds the lowest electronegativity, it's essential to compare it to other elements with similarly low values. Cesium (Cs), another alkali metal situated directly above francium, also exhibits remarkably low electronegativity. However, francium's larger atomic radius and weaker effective nuclear charge give it the edge.
Other elements with low electronegativity include:
- Cesium (Cs): The alkali metal immediately above francium in the periodic table.
- Rubidium (Rb): Another alkali metal, slightly higher electronegativity than cesium.
- Potassium (K): Another alkali metal, further up the periodic table than rubidium.
These elements all share the common characteristics of:
- Belonging to Group 1 (Alkali Metals): Alkali metals possess only one valence electron, readily lost to achieve a stable octet, thus exhibiting low electronegativity.
- Large Atomic Radii: As we move down Group 1, the atomic radius increases, resulting in decreased electronegativity.
The Practical Implications of Low Electronegativity
The extremely low electronegativity of francium has practical implications, though limited due to its extreme rarity and radioactivity. Its behavior in chemical reactions is strongly influenced by its tendency to readily lose its single valence electron, forming ionic bonds with highly electronegative elements. This means that francium readily participates in oxidation-reduction reactions, acting as a strong reducing agent.
Challenges in Studying Francium:
The highly radioactive nature of francium poses significant challenges in its study. Its short half-life (around 22 minutes for the longest-lived isotope) limits the amount of time available for experimentation. The extreme reactivity of francium also adds to the difficulty in handling and analyzing this element.
Theoretical Significance:
Despite its practical limitations, francium's extremely low electronegativity holds significant theoretical importance in understanding the trends and relationships within the periodic table. It serves as a compelling example illustrating the effects of atomic radius, nuclear charge, and shielding on the chemical properties of elements.
Electronegativity Trends Across the Periodic Table: A Visual Guide
Understanding electronegativity trends is crucial for predicting the types of bonds formed between atoms. Generally:
- Electronegativity increases across a period (left to right): As we move across a period, the nuclear charge increases, while the atomic radius remains relatively constant, leading to an increase in electronegativity.
- Electronegativity decreases down a group (top to bottom): As we move down a group, the atomic radius increases significantly, and the shielding effect becomes more pronounced, causing a decrease in electronegativity.
This pattern is clearly visible in the periodic table. The most electronegative elements are found in the upper right corner (fluorine), while the least electronegative elements reside in the bottom left corner (francium).
Beyond Francium: Exploring Other Low Electronegativity Elements and their Applications
While francium holds the record for the lowest electronegativity, other elements with relatively low electronegativity have found practical applications:
- Cesium: Used in atomic clocks for precise timekeeping, due to its unique atomic properties. Also used in specialized photoelectric cells.
- Potassium: An essential nutrient for living organisms, playing a critical role in numerous biological processes. Also used in fertilizers.
- Sodium: Widely used in everyday applications, including table salt (NaCl), street lighting (sodium lamps), and various industrial processes.
These elements, while not possessing the lowest electronegativity, highlight the significance of low electronegativity in various technological and biological applications.
Conclusion: The Reign of Francium
In conclusion, francium (Fr) reigns supreme as the element with the lowest electronegativity. This property, stemming from its large atomic radius, weak effective nuclear charge, and low ionization energy, solidifies its position at the bottom-left corner of the periodic table and underscores the remarkable trends in electronegativity. While its extreme radioactivity limits its practical applications, francium’s low electronegativity remains a testament to the fascinating interplay of fundamental atomic properties and serves as a pivotal concept in understanding chemical bonding and reactivity. Further research into this elusive element may continue to reveal its unique properties and potential applications in the future.
Latest Posts
Latest Posts
-
What Is The Area Of Triangle Rst
Apr 08, 2025
-
New Ocean Crust Is Formed At
Apr 08, 2025
-
Why Is The Melting Of Ice Not A Chemical Reaction
Apr 08, 2025
-
How To Find Mole From Volume
Apr 08, 2025
-
Find The Derivative Of The Function Y 5x 5x 5x
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about Which Is The Element With The Lowest Electronegativity . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.