Which Element Has Highest Ionization Energy
listenit
Mar 19, 2025 · 5 min read
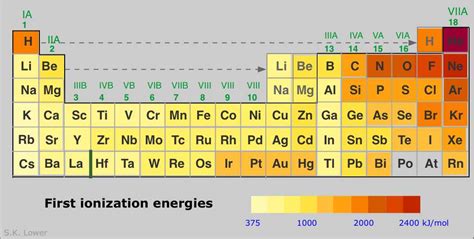
Table of Contents
Which Element Has the Highest Ionization Energy? Unraveling the Trends in Atomic Behavior
The quest to identify the element boasting the highest ionization energy takes us on a fascinating journey through the intricacies of atomic structure and periodic trends. Ionization energy, a fundamental concept in chemistry, quantifies the energy required to remove an electron from a gaseous atom or ion. This seemingly simple process is deeply intertwined with factors like nuclear charge, electron shielding, and atomic radius, all of which conspire to determine an element's tenacity in holding onto its electrons. Understanding these factors is key to deciphering which element reigns supreme in ionization energy.
Understanding Ionization Energy: A Deeper Dive
Before we pinpoint the champion, let's solidify our understanding of ionization energy itself. It's crucial to remember that ionization energy is not a single value but rather a series of values, representing the energy needed to remove successive electrons. The first ionization energy (IE₁) refers to the energy required to remove the outermost, or valence, electron. Subsequent ionization energies (IE₂, IE₃, and so on) represent the energy needed to remove subsequent electrons, each increasingly closer to the nucleus. This means that subsequent ionization energies are always greater than the preceding ones.
The process of ionization can be represented by the following equation:
X(g) + energy → X⁺(g) + e⁻
Where:
- X(g) represents the neutral gaseous atom.
- X⁺(g) represents the resulting gaseous ion with a +1 charge.
- e⁻ represents the removed electron.
Periodic Trends and Ionization Energy: The Guiding Principles
The periodic table is not just a neatly organized list of elements; it's a powerful visual representation of periodic trends. These trends, including ionization energy, are directly linked to the arrangement of electrons in atoms.
1. Nuclear Charge: The Stronger the Pull, the Higher the Energy
The positive charge of the nucleus exerts an attractive force on the negatively charged electrons. A greater nuclear charge implies a stronger pull on the electrons, making it harder to remove them and thus leading to a higher ionization energy. As we move across a period (from left to right) on the periodic table, the nuclear charge increases, resulting in a general increase in ionization energy.
2. Electron Shielding: The Protective Layer
Inner electrons act as a shield, partially neutralizing the attractive force of the nucleus on the outer electrons. This shielding effect reduces the effective nuclear charge experienced by the valence electrons. As we move down a group (from top to bottom) on the periodic table, the number of inner electrons increases, enhancing shielding and reducing the effective nuclear charge. This leads to a decrease in ionization energy.
3. Atomic Radius: Distance Matters
The distance between the nucleus and the valence electrons, also known as the atomic radius, plays a significant role. A larger atomic radius means the valence electrons are farther from the nucleus and experience a weaker attractive force, resulting in lower ionization energy. Conversely, a smaller atomic radius leads to a stronger attractive force and higher ionization energy. Atomic radius generally increases as we move down a group and decreases as we move across a period.
Identifying the Element with the Highest Ionization Energy: Helium's Reign
Considering the interplay of nuclear charge, shielding, and atomic radius, we can now confidently pinpoint the element with the highest first ionization energy: Helium (He).
Helium's position in the periodic table is paramount to its high ionization energy. Located in the top right corner of the table (excluding noble gases), it possesses a relatively small atomic radius and a strong nuclear charge. With only two electrons, both of which are in the first electron shell, there's minimal electron shielding. The close proximity of the electrons to the nucleus and the strong pull from the +2 charge make it incredibly difficult to remove an electron. This translates to the highest first ionization energy amongst all elements.
It is important to note that subsequent ionization energies for Helium are significantly higher. This is because once the first electron is removed, the remaining electron experiences the full nuclear charge without any shielding.
Beyond Helium: Understanding Trends and Exceptions
While helium holds the crown for the highest first ionization energy, it's essential to explore the trends across the periodic table and consider subsequent ionization energies.
Noble Gases: The Reluctant Electron Donors
All noble gases exhibit high ionization energies. Their stable electron configurations (full outermost electron shells) make them extremely resistant to losing electrons. While helium has the highest first ionization energy, the heavier noble gases (neon, argon, krypton, xenon, and radon) also exhibit significantly high ionization energies, although progressively lower than helium.
Exceptions and Irregularities: The Nuances of Electron Configuration
Although periodic trends offer a general framework, there are subtle exceptions and irregularities. These often stem from electron configurations and subtle variations in electron-electron repulsions. For example, slight deviations from the expected trend may occur due to half-filled or completely filled subshells, which exhibit extra stability.
Applications and Significance of Ionization Energy
The concept of ionization energy isn't confined to theoretical chemistry; it has far-reaching practical applications. It plays a crucial role in several areas, including:
- Spectroscopy: Analyzing the light emitted or absorbed by atoms when electrons transition between energy levels directly relates to ionization energies.
- Materials Science: Understanding ionization energy is critical for designing materials with specific electrical and chemical properties.
- Analytical Chemistry: Ionization techniques are used in various analytical methods for identifying and quantifying elements.
- Astrophysics: Studying the spectra of stars and other celestial bodies involves analyzing ionization energies to determine their elemental composition.
Conclusion: Helium's Dominance and the Broader Picture
In conclusion, Helium (He) unequivocally holds the title for the highest first ionization energy. This is a direct consequence of its unique atomic structure – a small atomic radius, a strong nuclear charge, and minimal electron shielding. Understanding ionization energy and the periodic trends that govern it is fundamental to appreciating the fascinating interplay of atomic structure and chemical behavior. While helium stands out for its exceptional first ionization energy, the concept extends far beyond a single element, shaping diverse scientific fields and revealing the underlying principles of atomic interactions. The journey to understand ionization energy is a continuous exploration of the intricate world of atomic physics and chemistry.
Latest Posts
Latest Posts
-
Is Mars A Terrestrial Or Gaseous Planet
May 09, 2025
-
Where Does Transcription Occur In A Prokaryotic Cell
May 09, 2025
-
The Sum Of A Number And 3
May 09, 2025
-
What Is The Name Of The Molecular Compound So3
May 09, 2025
-
What Is 70 F To Celsius
May 09, 2025
Related Post
Thank you for visiting our website which covers about Which Element Has Highest Ionization Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.