What's The Atomic Number For Helium
listenit
Mar 29, 2025 · 6 min read
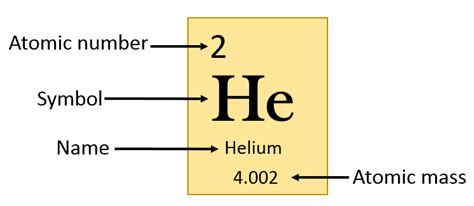
Table of Contents
What's the Atomic Number for Helium? A Deep Dive into the Noble Gas
Helium, the second lightest element in the universe, is a fascinating subject for scientists and enthusiasts alike. Its unique properties, from its low density to its role in various technological applications, make it a key element to understand. But before we delve into its intriguing characteristics, let's answer the fundamental question: what is the atomic number for helium?
The Atomic Number: 2
The atomic number for helium is 2. This seemingly simple number holds a wealth of information about the element's structure and behavior. The atomic number represents the number of protons in an atom's nucleus. Since atoms are electrically neutral, the number of protons also equals the number of electrons orbiting the nucleus. Therefore, a helium atom possesses two protons and two electrons.
Understanding Atomic Structure
To fully grasp the significance of helium's atomic number, let's briefly revisit the basics of atomic structure:
- Protons: Positively charged particles residing in the atom's nucleus. The number of protons defines the element.
- Neutrons: Neutral particles also found in the nucleus. Isotopes of an element differ in the number of neutrons.
- Electrons: Negatively charged particles orbiting the nucleus in energy levels or shells. The arrangement of electrons determines the element's chemical properties.
Helium's two protons dictate its position on the periodic table, firmly placing it as the second element. This positioning also hints at its chemical inertness, a consequence of its electron configuration.
Helium's Electron Configuration and Chemical Inertness
Helium's two electrons completely fill its first electron shell (also known as the K-shell). A full outer electron shell provides exceptional stability, making helium incredibly unreactive. This chemical inertness is characteristic of the noble gases, a group of elements in the periodic table's 18th column. Helium's stable electron configuration is the primary reason why it doesn't readily form chemical bonds with other elements.
Noble Gases: A Family of Inert Elements
Helium belongs to the noble gas family, also known as inert gases. These elements are renowned for their reluctance to participate in chemical reactions. This inertness stems from their complete outer electron shells, making them highly stable and resistant to forming chemical bonds. Other noble gases include neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn).
Implications of Chemical Inertness
Helium's chemical inertness is crucial for many of its applications. For example, its non-reactivity makes it ideal for:
- Inflating balloons and airships: Unlike hydrogen, which is highly flammable, helium is safe to use in applications requiring buoyancy.
- Protecting sensitive equipment: Inert atmospheres created with helium prevent oxidation and other chemical reactions that could damage delicate instruments or materials.
- Deep-sea diving: Helium-oxygen mixtures are used in deep-sea diving to prevent decompression sickness ("the bends").
Helium's Unique Physical Properties
Beyond its atomic structure and chemical inertness, helium possesses several unique physical properties directly linked to its atomic number and electron configuration:
- Low Density: Helium is the second lightest element, only surpassed by hydrogen. This low density is responsible for its buoyancy.
- Low Boiling Point: Helium has the lowest boiling point of all elements, meaning it remains a gas even at extremely low temperatures. This makes it invaluable in cryogenics.
- High Thermal Conductivity: Helium conducts heat efficiently, making it useful in applications requiring precise temperature control.
- Superfluidity: At extremely low temperatures (near absolute zero), helium exhibits superfluidity, a state of matter characterized by zero viscosity and unusual quantum mechanical properties.
Helium's Applications: A Diverse Range
Helium's unique properties have led to its widespread use in a variety of fields:
- Cryogenics: Helium's low boiling point makes it essential for cooling superconducting magnets used in MRI machines, particle accelerators, and other scientific instruments.
- Welding: Helium is used as a shielding gas in welding to prevent oxidation and maintain the integrity of the weld.
- Leak Detection: Helium's small atomic size and inertness allow it to be used as a tracer gas to detect leaks in various systems.
- Medical Applications: Helium is used in MRI machines and in respiratory treatments for patients with breathing difficulties.
Helium: Abundance and Scarcity
While helium is relatively abundant in the universe (it's the second most abundant element after hydrogen), its terrestrial abundance is far lower. Most helium on Earth is formed by the radioactive decay of heavier elements.
Helium Extraction and Conservation
The primary source of helium for commercial use is natural gas. However, extracting helium from natural gas is an energy-intensive process, and the amount of helium found in natural gas varies widely geographically.
The scarcity of helium has become a growing concern. Its unique properties and widespread applications have increased demand, leading to calls for more sustainable extraction practices and conservation efforts.
Isotopes of Helium: Variations on a Theme
While the atomic number defines helium, it's important to note that different isotopes of helium exist. Isotopes are atoms of the same element with different numbers of neutrons. The most common isotopes of helium are:
- Helium-4 (⁴He): This is the most abundant isotope, making up over 99% of naturally occurring helium. It contains two protons and two neutrons.
- Helium-3 (³He): This isotope is much rarer, containing two protons and only one neutron. It has applications in nuclear fusion research.
Both isotopes still possess two protons, maintaining helium's atomic number of 2. The differences in neutron number lead to slight variations in mass and some nuclear properties but don’t significantly alter helium's characteristic chemical inertness.
The Future of Helium: Challenges and Opportunities
Helium's unique combination of properties and limited availability presents both challenges and opportunities. Continued research into helium extraction, conservation, and alternative materials is crucial to ensure sustainable access to this invaluable element. The development of new technologies that reduce helium consumption or utilize alternative materials could help mitigate future shortages.
Conclusion: The Significance of Atomic Number 2
The atomic number 2 definitively identifies helium. This seemingly simple number encapsulates the fundamental characteristics of this unique element—its chemical inertness, its unusual physical properties, and its diverse applications across numerous scientific and technological fields. Understanding helium's atomic number provides a gateway to appreciating the complexities of atomic structure and the multifaceted role this element plays in our world. As we continue to explore the mysteries of the universe and develop new technologies, helium, with its atomic number of 2, will undoubtedly continue to be a critical component of scientific advancement and technological innovation.
Latest Posts
Latest Posts
-
14 Is 70 Of What Number
Mar 31, 2025
-
What Is Half Of 1 1 3 Cup In Cooking
Mar 31, 2025
-
3 And 1 2 As An Improper Fraction
Mar 31, 2025
-
What Is 60 Percent Of 200
Mar 31, 2025
-
What Is 40 Oz In Pounds
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What's The Atomic Number For Helium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
