What Type Of Bond Holds Dna Together
listenit
Mar 21, 2025 · 6 min read
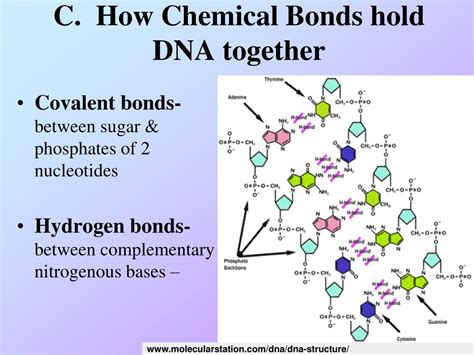
Table of Contents
What Type of Bond Holds DNA Together? A Deep Dive into the Molecular Interactions
The structure of DNA, that iconic double helix, is a marvel of nature. But what exactly holds this elegant structure together? The answer isn't as simple as a single type of bond, but rather a complex interplay of several forces. Understanding these interactions is crucial to grasping DNA's stability, its ability to replicate, and its overall function within the cell. This article will explore the various bonds and forces that maintain the integrity of the DNA molecule, delving into the details of each and their contribution to the overall stability of the genetic code.
The Backbone: Phosphodiester Bonds
The very foundation of the DNA structure lies in the phosphodiester bonds. These are strong covalent bonds that link the individual nucleotides together to form the sugar-phosphate backbone of each DNA strand. A nucleotide consists of a deoxyribose sugar, a phosphate group, and a nitrogenous base (adenine, guanine, cytosine, or thymine).
How Phosphodiester Bonds Form
The 3'-hydroxyl group (-OH) of one deoxyribose sugar reacts with the 5'-phosphate group of the next sugar. This reaction releases a water molecule and forms a phosphodiester linkage. This creates a continuous sugar-phosphate backbone running along the length of each DNA strand. The directionality of this backbone (5' to 3') is crucial for DNA replication and transcription.
Significance of Covalent Bonds
The covalent nature of phosphodiester bonds is paramount. Covalent bonds are strong chemical bonds formed by the sharing of electrons between atoms. This strength ensures the integrity of the DNA backbone, making it resistant to breakage under normal cellular conditions. This robust backbone provides a stable framework for the genetic information carried by the nitrogenous bases.
The Rungs of the Ladder: Hydrogen Bonds
While the phosphodiester bonds form the structural scaffolding of DNA, the interaction between the nitrogenous bases is responsible for the specificity of the genetic code and the formation of the iconic double helix. This interaction is mediated primarily by hydrogen bonds.
Base Pairing: A Specific Interaction
Hydrogen bonds are weaker than covalent bonds, yet they play a crucial role in maintaining the double-stranded structure of DNA. They are formed between complementary base pairs: adenine (A) with thymine (T), and guanine (G) with cytosine (C).
- A-T base pair: forms two hydrogen bonds.
- G-C base pair: forms three hydrogen bonds.
This specific base pairing is essential for accurate DNA replication and transcription. The hydrogen bonds allow the two strands to be held together, but are weak enough to be broken during processes requiring strand separation, like DNA replication.
The Significance of Hydrogen Bond Strength
The difference in the number of hydrogen bonds between A-T and G-C base pairs influences the stability of the DNA molecule. G-C base pairs, with three hydrogen bonds, are generally stronger than A-T base pairs, with only two. Regions of DNA with a higher G-C content are thus more stable and require more energy to denature (separate the strands). This property is exploited in various molecular biology techniques.
Beyond Hydrogen Bonds: Other Intermolecular Forces
While hydrogen bonds are the primary force holding the base pairs together, several other intermolecular forces contribute to the overall stability of the DNA double helix. These include:
Van der Waals Forces
These weak, short-range attractive forces arise from temporary fluctuations in electron distribution around atoms and molecules. While individually weak, the cumulative effect of many van der Waals interactions between the stacked base pairs contributes significantly to the overall stability of the DNA helix. These interactions contribute to the stacking energy that stabilizes the double helix.
Hydrophobic Interactions
The nitrogenous bases are relatively hydrophobic (water-repelling), while the sugar-phosphate backbone is hydrophilic (water-attracting). This difference in polarity leads to hydrophobic interactions between the stacked base pairs, further contributing to the stability of the DNA double helix. The bases tend to cluster together in the interior of the helix, away from the surrounding aqueous environment.
Base Stacking: A Key Stabilizing Force
Base stacking is a critical aspect of DNA stability and is a consequence of several forces, including van der Waals forces, hydrophobic interactions, and pi-pi stacking interactions between the aromatic rings of the bases. The bases are stacked upon one another in a specific orientation, optimizing these interactions and contributing substantially to the helix's stability. The planar structure of the bases facilitates efficient stacking, enhancing the stability of the overall structure.
DNA Structure and Function: A Synergistic Relationship
The various bonds and forces discussed above act in concert to maintain the unique double-helical structure of DNA. This structure is not simply a static entity; it is intimately linked to DNA's function. The specific base pairing enables accurate replication and transcription, while the overall stability of the double helix protects the genetic information from damage.
The Importance of Flexibility
While stability is crucial, DNA also needs to be flexible enough to allow for the unwinding and rewinding of the double helix during replication, transcription, and other cellular processes. The combination of strong covalent bonds in the backbone and weaker hydrogen bonds between base pairs provides this necessary balance between stability and flexibility.
The Role of DNA Supercoiling
DNA is often found in a supercoiled state, which further contributes to its compaction and stability within the cell. This supercoiling involves twisting the double helix upon itself, effectively reducing the space it occupies. This compaction is essential for packaging the enormous length of DNA found in eukaryotic cells into the relatively small nucleus.
Proteins and DNA Interactions
The DNA structure isn't isolated; it interacts dynamically with a variety of proteins. These proteins are involved in DNA replication, transcription, repair, and other essential processes. Many of these protein-DNA interactions involve specific recognition of the DNA sequence, often mediated by hydrogen bonds and other weak interactions. This interaction demonstrates the intricate interplay between DNA structure and cellular function.
Conclusion: A Multifaceted Approach to Stability
The question of what holds DNA together is best answered not by a single type of bond but by a complex interplay of several forces. The strong covalent phosphodiester bonds provide the backbone, while the specific hydrogen bonding between base pairs dictates the genetic code and allows for strand separation. Van der Waals forces, hydrophobic interactions, and base stacking contribute significantly to the overall stability and compaction of the double helix. This intricate interplay of different types of interactions ensures that DNA's structure is stable enough to protect the genetic information, yet flexible enough to allow for the crucial cellular processes that depend on its accessibility. Understanding this multifaceted approach to DNA stability is crucial to appreciating the complexity and elegance of this fundamental molecule of life. Further research continues to uncover the nuances of these interactions and their implications for DNA function and regulation within the cell. The beauty of DNA lies not only in its simplicity of its base-pair structure but in the intricacy of the forces that support this iconic building block of life.
Latest Posts
Latest Posts
-
180 Inches Is How Many Yards
Mar 28, 2025
-
1 2x 1 2x X 1
Mar 28, 2025
-
What Is 7 20 As A Percent
Mar 28, 2025
-
What Element Is Shiny And Conducts Heat And Electricity
Mar 28, 2025
-
3x 2y 6 In Slope Intercept Form
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about What Type Of Bond Holds Dna Together . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
