What Is The Polymer Of Amino Acids
listenit
Mar 23, 2025 · 6 min read
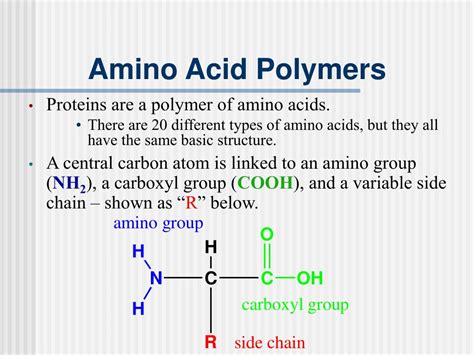
Table of Contents
What is the Polymer of Amino Acids? A Deep Dive into Proteins
Proteins are the workhorses of life, involved in virtually every biological process imaginable. From catalyzing reactions to providing structural support, their diverse functions are a testament to their intricate structure. But at the heart of this complexity lies a simple truth: proteins are polymers of amino acids. This article will delve deep into this fundamental concept, exploring the structure of amino acids, the process of polymerization, the different levels of protein structure, and the profound implications of protein structure and function.
Understanding Amino Acids: The Building Blocks of Proteins
Amino acids are the monomeric units that link together to form protein polymers. These organic molecules are characterized by a central carbon atom (the α-carbon) bonded to four different groups:
- An amino group (-NH₂): This group is basic, meaning it can accept a proton (H⁺).
- A carboxyl group (-COOH): This group is acidic, meaning it can donate a proton (H⁺).
- A hydrogen atom (-H): This simple group contributes to the overall structure.
- A side chain (R group): This group is unique to each amino acid and determines its chemical properties. The R group can be nonpolar, polar, acidic, or basic, significantly influencing the protein's overall properties and function.
There are 20 standard amino acids that are commonly incorporated into proteins during translation. These amino acids are categorized based on their R group properties:
Types of Amino Acid Side Chains:
-
Nonpolar, aliphatic: These amino acids have hydrocarbon side chains, making them hydrophobic (water-repelling). Examples include glycine, alanine, valine, leucine, isoleucine, and methionine.
-
Aromatic: These amino acids possess aromatic rings in their side chains, often contributing to the protein's stability and absorption of ultraviolet light. Examples include phenylalanine, tyrosine, and tryptophan.
-
Polar, uncharged: These amino acids have polar but uncharged side chains, making them hydrophilic (water-attracting). Examples include serine, threonine, cysteine, asparagine, and glutamine. Cysteine is unique due to its thiol (-SH) group, capable of forming disulfide bonds, crucial for protein structure.
-
Positively charged (basic): These amino acids have positively charged side chains at physiological pH. Examples include lysine, arginine, and histidine.
-
Negatively charged (acidic): These amino acids have negatively charged side chains at physiological pH. Examples include aspartic acid and glutamic acid.
The diversity of these R groups is what allows proteins to exhibit such a wide range of functions. The sequence of amino acids in a protein, its primary structure, dictates its three-dimensional conformation and ultimately its function.
Peptide Bonds: Linking Amino Acids to Form Polypeptides
The polymerization of amino acids occurs through a process called dehydration synthesis (also known as condensation). The carboxyl group of one amino acid reacts with the amino group of another amino acid, releasing a water molecule and forming a peptide bond (an amide bond). This bond links the α-carbon of one amino acid to the nitrogen atom of the next, creating a chain of amino acids called a polypeptide.
The peptide bond possesses a partial double-bond character due to resonance, which restricts rotation around the bond and influences the overall conformation of the polypeptide chain. The amino acid sequence is written from the N-terminus (the amino group end) to the C-terminus (the carboxyl group end).
Levels of Protein Structure: From Linear Sequence to Functional 3D Shape
The functional properties of a protein are intimately linked to its three-dimensional structure. This structure is hierarchical, with four distinct levels:
1. Primary Structure: The Amino Acid Sequence
The primary structure is simply the linear sequence of amino acids in the polypeptide chain. This sequence is dictated by the genetic code and is crucial because it determines all higher levels of structure. Even a single amino acid change can drastically alter the protein's function, as seen in sickle cell anemia, caused by a single point mutation in the β-globin gene.
2. Secondary Structure: Local Folding Patterns
The primary structure begins to fold into local patterns called secondary structures, primarily stabilized by hydrogen bonds between the backbone atoms (not the side chains). Common secondary structures include:
-
α-helices: These are right-handed coiled structures, stabilized by hydrogen bonds between the carbonyl oxygen of one amino acid and the amide hydrogen of an amino acid four residues away.
-
β-sheets: These are extended structures formed by hydrogen bonds between adjacent polypeptide chains (or segments of the same chain). β-sheets can be parallel (chains running in the same direction) or antiparallel (chains running in opposite directions).
-
Loops and turns: These irregular structures connect α-helices and β-sheets.
3. Tertiary Structure: The Overall 3D Arrangement
Tertiary structure refers to the overall three-dimensional arrangement of a polypeptide chain, including its secondary structures. This structure is stabilized by a variety of interactions between the side chains (R groups):
-
Hydrophobic interactions: Nonpolar side chains cluster together in the protein's interior, away from water.
-
Hydrogen bonds: Hydrogen bonds between polar side chains contribute to stability.
-
Ionic bonds (salt bridges): These are electrostatic interactions between oppositely charged side chains.
-
Disulfide bonds: Covalent bonds between cysteine residues, forming strong cross-links.
The tertiary structure defines the protein's overall shape and determines its function. Many proteins function as monomers, meaning they consist of a single polypeptide chain.
4. Quaternary Structure: Multiple Polypeptide Chains
Some proteins consist of multiple polypeptide chains, each with its own tertiary structure. The arrangement of these subunits is called the quaternary structure. These subunits can be identical or different. Hemoglobin, for example, is a tetramer (four subunits) consisting of two α-globin and two β-globin subunits.
Protein Folding and Chaperones
The process of protein folding is complex and not yet fully understood. However, it is known that the primary structure contains the information needed to guide the polypeptide chain to its correct tertiary structure. This process is often facilitated by molecular chaperones, proteins that assist in the proper folding of other proteins, preventing aggregation and misfolding. Misfolded proteins can lead to a variety of diseases, including Alzheimer's disease and Parkinson's disease.
The Importance of Protein Structure and Function
The relationship between protein structure and function is undeniable. The specific three-dimensional arrangement of a protein dictates its ability to interact with other molecules, whether it's binding to a substrate (in enzymes), transporting oxygen (in hemoglobin), or providing structural support (in collagen). Any alteration in the protein's structure, caused by mutations, environmental changes, or other factors, can impair its function and have severe consequences.
Conclusion: Proteins – The Marvelous Polymers of Amino Acids
Proteins, the polymers of amino acids, are fundamental to life's processes. Their intricate structures, from the linear sequence of amino acids to the complex three-dimensional arrangements, are meticulously crafted to perform a staggering array of functions. Understanding the structure and function of proteins is crucial for advancements in medicine, biotechnology, and numerous other scientific fields. Further research into protein folding, misfolding, and the impact of mutations continues to unveil the remarkable complexity and importance of these biological macromolecules. The ongoing study of these amazing polymers will undoubtedly continue to reveal more insights into the mechanisms of life itself.
Latest Posts
Latest Posts
-
What Three Phases Of The Cell Cycle Are Considered Interphase
Mar 25, 2025
-
How Many Lines Of Symmetry Are In A Rectangle
Mar 25, 2025
-
How To Find 2 3 Of A Number
Mar 25, 2025
-
What Is The Gcf Of 24 And 40
Mar 25, 2025
-
What Is 80 In A Fraction
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about What Is The Polymer Of Amino Acids . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
