What Is The Ph At The Equivalence Point
listenit
Mar 19, 2025 · 5 min read
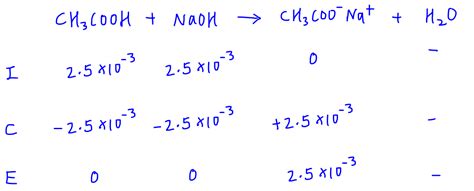
Table of Contents
What is the pH at the Equivalence Point? A Comprehensive Guide
Determining the pH at the equivalence point of a titration is crucial for understanding acid-base chemistry and performing accurate quantitative analyses. The equivalence point itself represents the stoichiometric point in a titration where the moles of titrant added are exactly equal to the moles of analyte present. However, the pH at this point isn't always 7, as many assume. This comprehensive guide will delve into the factors that influence the pH at the equivalence point and provide methods for its calculation and prediction.
Understanding Titration and the Equivalence Point
Titration is a quantitative analytical technique used to determine the concentration of an unknown solution (analyte) by reacting it with a solution of known concentration (titrant). The reaction is typically an acid-base neutralization, where a strong acid reacts with a strong base, or vice versa. The equivalence point signifies the exact moment when the moles of acid and base are chemically equivalent, meaning they have completely neutralized each other.
Key Concepts:
- Analyte: The substance whose concentration is being determined.
- Titrant: The solution of known concentration added to the analyte.
- Equivalence Point: The point in the titration where the moles of titrant added are exactly equal to the moles of analyte.
- Endpoint: The point in the titration where a visual indicator changes color, signaling the equivalence point (ideally, the endpoint closely approximates the equivalence point).
pH at the Equivalence Point: It's Not Always 7!
The common misconception that the pH at the equivalence point is always 7 stems from the neutralization of a strong acid with a strong base. In this case, the resulting solution contains only water and a salt, neither of which significantly impacts the pH, resulting in a neutral pH of 7 at 25°C.
However, the pH at the equivalence point varies significantly depending on the strength of the acid and base involved:
- Strong Acid - Strong Base: pH = 7 (at 25°C)
- Strong Acid - Weak Base: pH < 7 (acidic)
- Weak Acid - Strong Base: pH > 7 (basic)
- Weak Acid - Weak Base: pH depends on the relative strengths of the acid and base and requires more complex calculations.
Factors Affecting pH at the Equivalence Point
Several factors contribute to the deviation from a pH of 7 at the equivalence point:
-
Strength of the Acid and Base: The most significant factor. Strong acids and bases completely dissociate, while weak acids and bases only partially dissociate. This affects the concentration of H⁺ and OH⁻ ions in the solution at the equivalence point.
-
Concentration of the Solutions: Higher concentrations generally lead to a sharper change in pH near the equivalence point, making it easier to determine.
-
Temperature: Temperature affects the dissociation constant (Ka or Kb) of weak acids and bases, thus influencing the pH at the equivalence point.
-
Ionic Strength: The presence of ions in the solution can affect the activity of H⁺ and OH⁻ ions, slightly altering the pH.
-
Hydrolysis: The process where a salt reacts with water to produce acidic or basic solutions. This is particularly relevant when titrating weak acids or bases.
Calculating the pH at the Equivalence Point
The calculation method depends on the type of acid-base titration:
1. Strong Acid - Strong Base Titration
The pH at the equivalence point is 7 (at 25°C) due to the complete neutralization. No further calculations are required.
2. Strong Acid - Weak Base Titration
At the equivalence point, the solution contains the conjugate acid of the weak base. The pH is calculated using the Ka of the conjugate acid:
-
Determine the concentration of the conjugate acid: This is equal to the initial concentration of the weak base divided by the total volume at the equivalence point.
-
Calculate the [H⁺] using the Ka expression: Ka = [H⁺][A⁻]/[HA], where HA is the conjugate acid. Solve for [H⁺].
-
Calculate the pH: pH = -log[H⁺]
3. Weak Acid - Strong Base Titration
At the equivalence point, the solution contains the conjugate base of the weak acid. The pH is calculated using the Kb of the conjugate base:
-
Determine the concentration of the conjugate base: This is equal to the initial concentration of the weak acid divided by the total volume at the equivalence point.
-
Calculate the [OH⁻] using the Kb expression: Kb = [OH⁻][HA]/[A⁻], where A⁻ is the conjugate base. Solve for [OH⁻].
-
Calculate the pOH: pOH = -log[OH⁻]
-
Calculate the pH: pH = 14 - pOH (at 25°C)
4. Weak Acid - Weak Base Titration
This case is the most complex. The pH at the equivalence point depends on the relative strengths of the weak acid and weak base. The calculation involves considering the equilibrium of both the conjugate acid and conjugate base, often requiring iterative methods or numerical solvers.
Practical Applications and Importance
Understanding the pH at the equivalence point is crucial for numerous applications:
-
Quantitative Analysis: Precisely determining the concentration of unknown solutions in various fields like chemistry, environmental science, and medicine.
-
Pharmaceutical Industry: Ensuring the accurate concentration and purity of drugs.
-
Food and Beverage Industry: Monitoring the acidity or alkalinity of products.
-
Environmental Monitoring: Assessing water quality and pollutant levels.
Selecting the Right Indicator
The choice of indicator for a titration is crucial for accurate results. An indicator should change color close to the equivalence point. The pH range over which the indicator changes color should encompass the pH at the equivalence point. For example:
- Strong Acid - Strong Base: Phenolphthalein or bromthymol blue.
- Strong Acid - Weak Base: Methyl orange.
- Weak Acid - Strong Base: Phenolphthalein.
Conclusion
Determining the pH at the equivalence point is a fundamental aspect of acid-base titrations. While the simplistic notion of a pH of 7 is only true for strong acid-strong base titrations, understanding the factors influencing pH, particularly the strength of the acid and base, is critical for accurate calculations and experimental success. The methods outlined above provide a roadmap for calculating the pH at the equivalence point for different types of titrations, emphasizing the importance of this concept in various scientific and industrial applications. The selection of a suitable indicator is also a key factor in obtaining accurate and reliable titration results. By mastering these concepts, one can confidently perform and interpret acid-base titrations with a deeper understanding of the underlying chemistry.
Latest Posts
Latest Posts
-
What Is Half Of 1 5 Teaspoons
Mar 19, 2025
-
12 Is 40 Of What Number
Mar 19, 2025
-
Where Is Rna Found In Cell
Mar 19, 2025
-
What Is 6 Percent Of 15
Mar 19, 2025
-
What Is The Square Root Of 162
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about What Is The Ph At The Equivalence Point . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
