What Is The Number Of Valence Electrons In Oxygen
listenit
Mar 15, 2025 · 6 min read
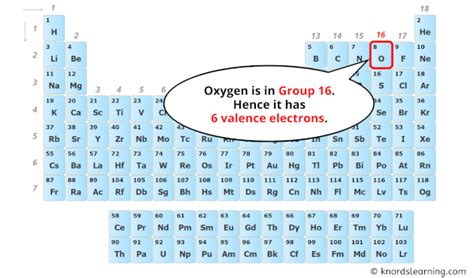
Table of Contents
What is the Number of Valence Electrons in Oxygen? A Deep Dive into Atomic Structure and Bonding
Oxygen, a life-sustaining element crucial for respiration and countless chemical processes, holds a significant place in the periodic table. Understanding its atomic structure, particularly the number of valence electrons, is key to grasping its chemical behavior and reactivity. This article delves deep into the concept of valence electrons, exploring oxygen's electronic configuration and its implications for bonding, oxidation states, and overall chemical properties. We will also explore how this knowledge is fundamental to various scientific fields.
Understanding Valence Electrons: The Key to Chemical Reactivity
Valence electrons are the outermost electrons in an atom. These electrons are the ones involved in chemical bonding, determining an element's reactivity and the types of bonds it can form. They occupy the highest energy level (principal quantum shell) in an atom's electron configuration. The number of valence electrons dictates an atom's tendency to gain, lose, or share electrons to achieve a stable electron configuration, usually a full outermost shell, often resembling the electronic configuration of a noble gas. This stable configuration, often referred to as the octet rule (eight electrons in the outermost shell), drives many chemical reactions.
Oxygen's Electronic Configuration: Unveiling the Valence Electrons
Oxygen's atomic number is 8, meaning it has 8 protons and 8 electrons in a neutral atom. To determine the number of valence electrons, we must examine its electronic configuration. Using the Aufbau principle and Hund's rule, we can fill the electron orbitals systematically:
- 1s² 2s² 2p⁴
This configuration tells us:
- 1s²: Two electrons occupy the first energy level (n=1) in the 1s orbital.
- 2s²: Two electrons occupy the second energy level (n=2) in the 2s orbital.
- 2p⁴: Four electrons occupy the second energy level (n=2) in the 2p orbitals (2px, 2py, 2pz).
Since the valence electrons are the electrons in the outermost shell (n=2 in this case), oxygen has six valence electrons. These six electrons are located in the 2s and 2p orbitals.
Oxygen's Reactivity: A Consequence of Six Valence Electrons
Oxygen's six valence electrons make it highly reactive. To achieve a stable octet, oxygen has a strong tendency to gain two electrons, forming an oxide ion (O²⁻) with a stable noble gas configuration similar to neon (1s² 2s² 2p⁶). This electron gain is why oxygen readily forms covalent bonds, sharing electrons with other atoms to complete its octet, or ionic bonds, accepting electrons from highly electropositive metals.
Covalent Bonding in Oxygen: Sharing is Caring
Oxygen frequently forms covalent bonds, sharing electron pairs with other atoms. A prime example is the oxygen molecule (O₂), where two oxygen atoms share two pairs of electrons, creating a double bond. This double bond is strong, contributing to the relatively high stability of diatomic oxygen in the atmosphere. Oxygen also forms covalent bonds with numerous other elements, including hydrogen (in water, H₂O), carbon (in carbon dioxide, CO₂), and nitrogen (in various nitrogen oxides). The ability to form multiple covalent bonds stems directly from its six valence electrons, allowing it to share electrons in multiple bonding interactions.
Ionic Bonding in Oxygen: Electron Transfer and Oxide Formation
When oxygen interacts with highly electropositive metals like sodium (Na) or magnesium (Mg), it readily accepts electrons to form ionic bonds. The metals lose electrons, becoming positively charged cations (Na⁺ or Mg²⁺), while oxygen gains electrons, becoming a negatively charged oxide anion (O²⁻). This electron transfer leads to the formation of ionic compounds such as sodium oxide (Na₂O) and magnesium oxide (MgO), held together by electrostatic attraction between the oppositely charged ions. The strong electronegativity of oxygen drives this electron transfer.
Oxidation States and Oxygen's Role in Redox Reactions
The concept of valence electrons directly relates to oxidation states, a measure of an atom's apparent charge in a compound. Oxygen typically exhibits an oxidation state of -2 in its compounds. This is because it usually gains two electrons to complete its octet. However, exceptions exist, such as in peroxides (e.g., hydrogen peroxide, H₂O₂) where oxygen has an oxidation state of -1, and in superoxides (e.g., potassium superoxide, KO₂) where it has an oxidation state of -1/2. These exceptions highlight the nuanced nature of oxidation states and their dependence on the specific chemical environment. Oxygen's involvement in redox reactions (reduction-oxidation reactions) often centers around its tendency to accept electrons and gain a negative oxidation state, making it a strong oxidizing agent.
Oxygen's Importance Across Diverse Scientific Fields
The understanding of oxygen's valence electrons and its resultant chemical properties is crucial across numerous scientific disciplines:
-
Biology: Oxygen's role in respiration, a fundamental biological process, depends directly on its ability to accept electrons. The electron transfer chain in cellular respiration utilizes oxygen as the final electron acceptor, generating ATP, the energy currency of cells. Without oxygen's unique electronic structure, life as we know it would be impossible.
-
Chemistry: Oxygen's reactivity is central to many chemical reactions, including combustion, oxidation, and the formation of numerous compounds. Understanding its valence electrons is fundamental to predicting reaction outcomes and designing new materials and chemical processes.
-
Materials Science: Oxygen's ability to form strong bonds with various elements influences the properties of countless materials. Oxides, for example, exhibit diverse properties, ranging from electrically insulating to superconducting, making them essential components in various technologies.
-
Environmental Science: Oxygen's presence and distribution in the atmosphere and oceans significantly impact environmental processes. The oxygen cycle, involving photosynthesis and respiration, is crucial for maintaining the Earth's habitability. Understanding oxygen's chemical behavior is important for studying atmospheric pollution and climate change.
-
Geochemistry: Oxygen is a major component of the Earth's crust and mantle, present in numerous minerals and rocks. Its chemical interactions with other elements influence rock formation, weathering processes, and the Earth's overall geochemical cycle.
Conclusion: The Significance of Six Valence Electrons
Oxygen's six valence electrons are not merely a numerical fact; they are the fundamental drivers of its chemical behavior and its multifaceted roles in the natural world. Its reactivity, ability to form various types of bonds, and involvement in critical biological and geochemical processes all stem from this seemingly simple characteristic of its electronic structure. Understanding the number of valence electrons in oxygen and its consequences is therefore crucial for grasping its significance in various scientific domains and for advancing our understanding of the world around us. From the air we breathe to the rocks beneath our feet, oxygen's impact is undeniable, and its six valence electrons are at the heart of its influence.
Latest Posts
Latest Posts
-
What Percent Of 75 Is 40
Mar 17, 2025
-
Translating Graph Up By 4 Units
Mar 17, 2025
-
What Is The Least Common Multiple Of 3 2
Mar 17, 2025
-
What Is The Derivative Of Ln 1 X
Mar 17, 2025
-
Which Type Of Molecule Is Shown Below
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Is The Number Of Valence Electrons In Oxygen . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
