What Is The Name Of The Following Compound
listenit
Mar 22, 2025 · 6 min read
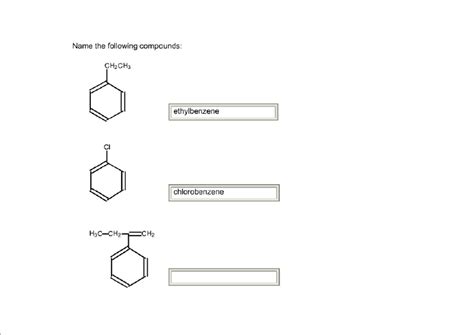
Table of Contents
I cannot answer the question "What is the name of the following compound?" without knowing the compound's chemical structure or formula. To provide a comprehensive and SEO-optimized blog post, I need that information. However, I can create a sample blog post about naming organic compounds, which will cover various aspects of nomenclature and serve as a template you can adapt once you provide the compound's details.
Decoding the Language of Chemistry: A Comprehensive Guide to Organic Compound Naming
Naming organic compounds might seem like a daunting task, a cryptic language spoken only by seasoned chemists. However, understanding the system is surprisingly straightforward once you grasp the fundamental rules and principles. This comprehensive guide will delve into the intricacies of organic nomenclature, equipping you with the knowledge to name a wide range of organic molecules confidently.
Understanding the IUPAC System
The International Union of Pure and Applied Chemistry (IUPAC) is the global authority on chemical nomenclature. The IUPAC system provides a standardized and unambiguous method for naming organic compounds, ensuring clarity and consistency across the scientific community. This system is based on a series of rules and priorities, which we'll explore in detail below.
Key Components of IUPAC Nomenclature
Several essential components are crucial for correctly naming an organic compound using the IUPAC system. These include:
- Identifying the Parent Chain: The longest continuous carbon chain within the molecule forms the parent chain. This chain determines the base name of the compound.
- Identifying Substituents: Any atoms or groups of atoms attached to the parent chain are considered substituents. These are named and their positions indicated.
- Numbering the Carbon Atoms: The carbon atoms in the parent chain are numbered to indicate the position of substituents. Numbering begins at the end of the chain that gives the substituents the lowest possible numbers.
- Alphabetical Ordering of Substituents: Substituents are listed alphabetically in the name, ignoring prefixes like "di," "tri," and "tetra."
- Using Prefixes to Indicate Multiple Substituents: If multiple identical substituents are present, prefixes like "di," "tri," "tetra," etc., are used to indicate their number.
Alkanes: The Foundation of Organic Nomenclature
Alkanes are the simplest hydrocarbons, consisting only of carbon and hydrogen atoms with single bonds. Understanding their nomenclature is fundamental to understanding the naming of more complex organic molecules.
Naming Straight-Chain Alkanes
Straight-chain alkanes have the general formula C<sub>n</sub>H<sub>2n+2</sub>, where 'n' represents the number of carbon atoms. The first four alkanes are methane (CH<sub>4</sub>), ethane (C<sub>2</sub>H<sub>6</sub>), propane (C<sub>3</sub>H<sub>8</sub>), and butane (C<sub>4</sub>H<sub>10</sub>). Longer chains use Greek prefixes to indicate the number of carbon atoms (e.g., pentane, hexane, heptane, octane, etc.).
Naming Branched-Chain Alkanes
Branched-chain alkanes contain carbon atoms branching off the main chain. Naming these requires:
- Identifying the longest continuous carbon chain: This chain forms the parent alkane.
- Identifying and naming the substituents: Alkyl groups (alkyl substituents) are named by replacing the "-ane" ending of the corresponding alkane with "-yl" (e.g., methyl, ethyl, propyl, butyl).
- Numbering the parent chain: Begin numbering at the end closest to the first substituent.
- Listing substituents alphabetically: Include the position number of each substituent.
Example: Consider a molecule with a five-carbon chain, a methyl group on carbon 2, and an ethyl group on carbon 3. The name would be 3-ethyl-2-methylpentane.
Alkenes and Alkynes: Incorporating Unsaturation
Alkenes contain at least one carbon-carbon double bond, while alkynes contain at least one carbon-carbon triple bond. These introduce additional complexity to the naming process.
Naming Alkenes
The naming of alkenes follows similar principles to alkanes, but with the addition of:
- Identifying the longest carbon chain containing the double bond: This chain forms the parent alkene.
- Numbering the carbon chain: Begin numbering at the end closest to the double bond, giving the double bond the lowest possible number.
- Indicating the position of the double bond: Use the number of the first carbon atom in the double bond as a prefix.
Example: A four-carbon chain with a double bond between carbons 1 and 2 would be named 1-butene.
Naming Alkynes
The naming of alkynes is analogous to alkenes, with the "-ene" suffix replaced by "-yne" to indicate the presence of a triple bond.
Functional Groups: Expanding the Complexity
Functional groups are specific groups of atoms within a molecule that confer characteristic chemical properties. Their presence significantly impacts the naming conventions. Some common functional groups include alcohols (-OH), aldehydes (-CHO), ketones (-C=O), carboxylic acids (-COOH), and amines (-NH<sub>2</sub>).
Naming Alcohols
Alcohols contain a hydroxyl group (-OH) attached to a carbon atom. The name is derived from the parent alkane, replacing the "-e" ending with "-ol." The position of the hydroxyl group is indicated by a number.
Naming Aldehydes and Ketones
Aldehydes contain a carbonyl group (-CHO) at the end of a carbon chain, while ketones contain a carbonyl group (-C=O) within the carbon chain. Aldehydes use the suffix "-al," while ketones use the suffix "-one."
Naming Carboxylic Acids
Carboxylic acids contain a carboxyl group (-COOH). Their names end in "-oic acid."
Naming Amines
Amines are derived from ammonia (NH<sub>3</sub>) by replacing one or more hydrogen atoms with alkyl or aryl groups. They are named by indicating the alkyl or aryl groups attached to the nitrogen atom, followed by the suffix "-amine".
Beyond the Basics: Advanced Nomenclature
The rules outlined above provide a foundation for naming a wide range of organic compounds. However, more complex molecules with multiple functional groups or cyclic structures require a deeper understanding of IUPAC rules and priorities. This often involves using prefixes and suffixes to denote specific functional groups and their positions within the molecule. Consulting IUPAC nomenclature guidelines is essential for accurately naming these more intricate structures.
Putting It All Together: A Practical Approach
Mastering organic nomenclature requires practice and careful attention to detail. Start with simpler molecules and gradually increase the complexity. Use online resources and textbooks to reinforce your understanding and familiarize yourself with the various functional groups and their naming conventions.
Conclusion: The Power of Precise Naming
Accurate naming of organic compounds is paramount in chemical communication. The IUPAC system ensures that scientists worldwide can unambiguously identify and discuss specific molecules, fostering collaboration and progress in the field. By understanding and applying the principles outlined in this guide, you can confidently decode the language of chemistry and participate in this vital aspect of scientific discourse. Remember, practice makes perfect! The more you work with naming organic compounds, the more intuitive the process becomes.
Latest Posts
Latest Posts
-
Difference Between A Sequence And Series
Mar 24, 2025
-
Are All Equilateral Triangles Isosceles Triangles
Mar 24, 2025
-
10 Is What Percent Of 200
Mar 24, 2025
-
What Is 0 85 As A Fraction
Mar 24, 2025
-
5 Out Of 25 Is What Percent
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about What Is The Name Of The Following Compound . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
