What Is The Electronic Configuration Of Potassium
listenit
Mar 17, 2025 · 5 min read
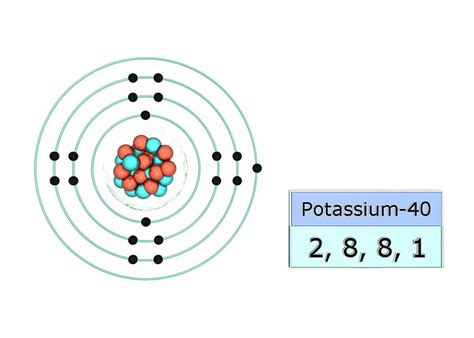
Table of Contents
What is the Electronic Configuration of Potassium? A Deep Dive into Atomic Structure
Potassium, a crucial element for life and a common component in fertilizers, holds a fascinating place in the periodic table. Understanding its electronic configuration is key to unlocking its unique chemical properties and behaviors. This article will delve deep into the electronic configuration of potassium, exploring its derivation, implications, and significance within the broader context of atomic structure and chemical bonding.
Understanding Electronic Configuration
Before diving into the specifics of potassium, let's establish a foundational understanding of electronic configuration. An electronic configuration describes the arrangement of electrons within an atom's electron shells and subshells. These arrangements dictate how an atom will interact with other atoms, forming chemical bonds and influencing its overall chemical reactivity. The configuration is typically represented using a notation that specifies the principal energy level (n), the subshell (s, p, d, or f), and the number of electrons in each subshell. For instance, 1s² represents two electrons in the 1s subshell.
The filling of electron subshells follows specific rules, primarily the Aufbau principle, the Pauli exclusion principle, and Hund's rule. The Aufbau principle states that electrons fill the lowest energy levels first. The Pauli exclusion principle dictates that no two electrons within an atom can have the same set of four quantum numbers (n, l, ml, and ms), meaning a maximum of two electrons can occupy a single orbital, with opposite spins. Finally, Hund's rule states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital.
Deriving the Electronic Configuration of Potassium (K)
Potassium (K) has an atomic number of 19, meaning it possesses 19 protons and, in its neutral state, 19 electrons. To determine its electronic configuration, we follow the Aufbau principle and the rules mentioned above:
-
First Shell (n=1): The first shell can only hold a maximum of two electrons in the 1s subshell. Therefore, we fill it completely:
1s² -
Second Shell (n=2): The second shell can hold a maximum of eight electrons, distributed across the 2s and 2p subshells. The 2s subshell fills first with two electrons (
2s²), followed by the 2p subshell, which can hold up to six electrons (2p⁶). This completes the second shell:2s²2p⁶ -
Third Shell (n=3): The third shell can hold up to 18 electrons. We continue filling this shell, starting with the 3s subshell which takes two electrons (
3s²), followed by the 3p subshell which holds another six electrons (3p⁶). At this point, we've accounted for 18 electrons (2 + 2 + 6 + 2 + 6 = 18). -
Fourth Shell (n=4): Potassium has 19 electrons. Therefore, the remaining electron occupies the 4s subshell:
4s¹.
Therefore, the complete electronic configuration of potassium is: 1s²2s²2p⁶3s²3p⁶4s¹.
This configuration is often shortened to [Ar]4s¹, where [Ar] represents the electronic configuration of Argon (1s²2s²2p⁶3s²3p⁶), the noble gas preceding potassium in the periodic table. This shorthand notation is commonly used to simplify the representation of electronic configurations for larger atoms.
Implications of Potassium's Electronic Configuration
Potassium's electronic configuration has profound implications for its chemical behavior and properties:
-
Reactivity: The single electron in the 4s subshell is relatively loosely held. This makes potassium highly reactive. It readily loses this electron to achieve a stable octet configuration, similar to the noble gas Argon. This tendency to lose an electron makes potassium a highly electropositive element.
-
Ionic Bonding: Because of its tendency to lose an electron, potassium readily forms ionic bonds with electronegative elements, such as chlorine (Cl), forming ionic compounds like potassium chloride (KCl). In this compound, potassium exists as a K⁺ ion, having lost its single valence electron.
-
Metallic Bonding: Potassium’s electronic configuration also contributes to its metallic properties. The loosely held valence electron can move freely between atoms, creating a "sea" of delocalized electrons. This electron mobility is responsible for potassium's good electrical and thermal conductivity.
-
Oxidation State: The most common oxidation state of potassium is +1, reflecting its tendency to lose one electron to achieve a stable noble gas configuration.
-
Biological Importance: The reactivity of potassium, specifically its ability to readily form ions, is crucial for its biological function. Potassium ions (K⁺) play vital roles in maintaining cell membrane potential, nerve impulse transmission, and muscle contraction.
Potassium's Position in the Periodic Table and its Electronic Configuration
Potassium's position in the periodic table as an alkali metal directly relates to its electronic configuration. All alkali metals have a single electron in their outermost s subshell, resulting in similar chemical properties. This shared characteristic leads to their high reactivity, ease of ionization, and formation of +1 ions. The electronic configuration provides a clear explanation for the observed trends and properties within the alkali metal group.
Further Exploration: Excited States and Ionization Energy
While the configuration discussed above represents the ground state of potassium, the atom can also exist in excited states. When an electron absorbs energy, it can jump to a higher energy level. These transitions are temporary, and the electron will eventually return to a lower energy level, emitting energy in the process (often as light).
The ionization energy of potassium, the energy required to remove its outermost electron, is relatively low, reflecting the ease with which potassium loses its valence electron. This low ionization energy is a direct consequence of its electronic configuration and the relatively weak attraction of the nucleus for the 4s electron.
Conclusion: The Significance of Understanding Potassium's Electronic Configuration
Understanding the electronic configuration of potassium is vital for comprehending its chemical properties, reactivity, and biological roles. This seemingly simple configuration—[Ar]4s¹—explains why potassium is a highly reactive alkali metal, readily forms ionic bonds, exhibits metallic properties, and plays a critical role in numerous biological processes. The principles used to derive potassium's electronic configuration apply to all elements, highlighting the fundamental importance of electronic structure in predicting and explaining the behavior of matter. The study of electronic configurations opens doors to a deeper appreciation of the intricacies of atomic structure and the remarkable diversity of chemical behavior observed in the periodic table.
Latest Posts
Latest Posts
-
What Is Square Root Of 45
Mar 18, 2025
-
What Is The Diameter Of A Tennis Ball
Mar 18, 2025
-
Solve This Equation H 9 7
Mar 18, 2025
-
Whats The Square Root Of 27
Mar 18, 2025
-
What Is The Difference Between Federalist And Anti Federalist
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electronic Configuration Of Potassium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
