What Is The Electron Configuration Of Phosphorus
listenit
Mar 16, 2025 · 5 min read
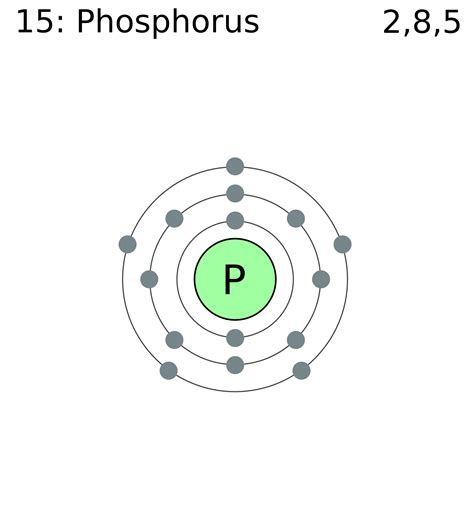
Table of Contents
What is the Electron Configuration of Phosphorus? A Deep Dive into Atomic Structure
Phosphorus, a crucial element for life and a key player in various industrial applications, possesses a fascinating electron configuration that dictates its chemical properties and reactivity. Understanding this configuration is fundamental to comprehending its behavior in different chemical environments. This comprehensive guide delves deep into the electron configuration of phosphorus, exploring its underlying principles, variations, and implications.
Understanding Electron Configuration
Before diving into phosphorus specifically, let's establish a foundational understanding of electron configuration. Electron configuration describes the arrangement of electrons within an atom's electron shells and subshells. It follows specific rules dictated by quantum mechanics, primarily the Aufbau principle, Hund's rule, and the Pauli exclusion principle.
- Aufbau Principle: Electrons fill the lowest energy levels first. This means that electrons occupy orbitals with the lowest possible energy before moving to higher energy levels.
- Hund's Rule: Within a subshell, electrons individually occupy each orbital before pairing up. This maximizes the total spin of the electrons.
- Pauli Exclusion Principle: No two electrons in an atom can have the same set of four quantum numbers (n, l, ml, and ms). This means that each orbital can hold a maximum of two electrons with opposite spins.
These principles guide us in predicting the electron configuration of any element, including phosphorus.
Determining the Electron Configuration of Phosphorus (P)
Phosphorus (P) has an atomic number of 15, meaning it has 15 protons and 15 electrons in a neutral atom. To determine its electron configuration, we follow the Aufbau principle and fill the orbitals sequentially:
1s², 2s², 2p⁶, 3s², 3p³
Let's break this down:
- 1s²: The first energy level (n=1) contains one subshell, the s subshell, which can hold a maximum of two electrons. Both electrons are paired in this orbital.
- 2s²: The second energy level (n=2) also has an s subshell, holding another two paired electrons.
- 2p⁶: The second energy level also contains the p subshell, which can hold up to six electrons (three orbitals, each holding two electrons). In phosphorus, all six electrons are present.
- 3s²: The third energy level (n=3) starts with an s subshell, filled with two paired electrons.
- 3p³: Finally, we reach the 3p subshell. Phosphorus has three electrons remaining, which occupy the three individual orbitals of the p subshell. According to Hund's rule, each orbital gets one electron before pairing starts.
Therefore, the complete electron configuration of phosphorus is 1s² 2s² 2p⁶ 3s² 3p³. This configuration highlights that phosphorus has three unpaired electrons in its outermost shell. This fact is crucial in understanding its chemical reactivity.
Orbital Diagrams and Phosphorus
To visualize the electron configuration more clearly, we can use orbital diagrams. These diagrams show the individual orbitals and the placement of electrons within them. For phosphorus, the orbital diagram would illustrate:
- 1s: ↑↑
- 2s: ↑↑
- 2p: ↑↑ ↑↑ ↑↑
- 3s: ↑↑
- 3p: ↑ ↑ ↑
The arrows represent electrons, and their direction indicates the spin. Note the three unpaired electrons in the 3p subshell, again emphasizing phosphorus's reactivity.
Phosphorus's Valence Electrons and Chemical Behavior
The outermost shell of electrons, called the valence shell, dictates an element's chemical behavior. In phosphorus, the valence shell is the third energy level (n=3), containing five electrons (two in the 3s subshell and three in the 3p subshell). These five valence electrons are responsible for phosphorus's ability to form various chemical bonds.
Phosphorus's tendency to gain or share electrons to achieve a stable octet (eight electrons in its valence shell) drives its reactivity. It frequently forms covalent bonds by sharing its three unpaired electrons in the 3p subshell, giving it a common valency of three (phosphine, PH₃, is a prime example). However, phosphorus can also expand its octet and exhibit valency of five, utilizing its 3d orbitals in bonding (e.g., phosphorus pentachloride, PCl₅).
Excited State Electron Configuration
The electron configuration discussed above represents phosphorus in its ground state, the lowest energy state. However, under specific conditions, such as absorbing energy (e.g., through heat or light), an electron can jump to a higher energy level, resulting in an excited state. For phosphorus, an electron from the 3p subshell could be promoted to the vacant 3d subshell, leading to an excited state electron configuration such as: 1s² 2s² 2p⁶ 3s² 3p² 3d¹. This excited state configuration influences phosphorus's ability to participate in different chemical reactions, particularly those requiring higher energy levels.
Isotopes and Electron Configuration
All isotopes of phosphorus have the same number of protons (15) and hence the same electron configuration in their neutral state. Isotopes differ only in the number of neutrons. The electron configuration remains unchanged as it is determined solely by the number of protons and electrons.
Phosphorus in Different Chemical Compounds
The electron configuration of phosphorus directly relates to its diverse chemistry and the types of compounds it forms. The presence of three unpaired electrons allows for the formation of numerous compounds with varying bonding characteristics.
Examples:
- Phosphine (PH₃): Phosphorus shares its three unpaired 3p electrons with three hydrogen atoms, forming three covalent bonds.
- Phosphoric Acid (H₃PO₄): Phosphorus forms multiple bonds with oxygen atoms, exhibiting a higher valency.
- Phosphorus Pentoxide (P₄O₁₀): A complex oxide where phosphorus displays a valency of five.
- Organophosphorus compounds: A vast class of compounds containing phosphorus-carbon bonds that plays significant roles in biology and industry. The variability of bonding stems directly from phosphorus's electron configuration.
Conclusion: The Significance of Phosphorus's Electron Configuration
The electron configuration of phosphorus, 1s² 2s² 2p⁶ 3s² 3p³, is not merely an abstract concept; it is the foundation of its chemical behavior. It governs its reactivity, its ability to form diverse compounds, and ultimately, its indispensable role in biological systems and industrial processes. Understanding this configuration provides a crucial framework for comprehending phosphorus's importance in various fields of study and application. The subtle yet profound implications of this seemingly simple configuration showcase the elegant power of quantum mechanics in explaining the behavior of the elements around us. Further exploration into the nuances of phosphorus's electronic structure opens doors to a deeper appreciation of chemistry and its vast applications. From the biological significance of phosphates in DNA to the industrial uses of phosphorus compounds in fertilizers and other materials, the fundamental atomic structure, dictated by its electron configuration, underlies its widespread impact.
Latest Posts
Latest Posts
-
Translating Graph Up By 4 Units
Mar 17, 2025
-
What Is The Least Common Multiple Of 3 2
Mar 17, 2025
-
What Is The Derivative Of Ln 1 X
Mar 17, 2025
-
Which Type Of Molecule Is Shown Below
Mar 17, 2025
-
How To Find The Domain Of F O G
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration Of Phosphorus . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
