What Is The Electron Configuration Of Germanium
listenit
Apr 07, 2025 · 6 min read
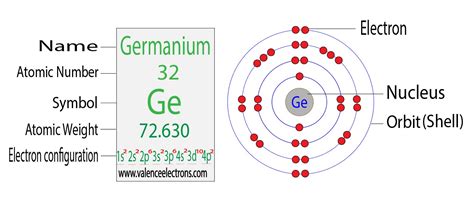
Table of Contents
What is the Electron Configuration of Germanium? A Deep Dive into Atomic Structure
Germanium, a metalloid element with fascinating properties, plays a crucial role in various technological applications, from semiconductors to fiber optics. Understanding its atomic structure, particularly its electron configuration, is key to grasping its unique characteristics and behavior. This comprehensive article delves deep into the electron configuration of germanium, exploring its implications for chemical bonding, conductivity, and overall properties.
Understanding Electron Configuration
Before diving into germanium's specifics, let's establish a foundational understanding of electron configuration. An electron configuration describes the arrangement of electrons in the various energy levels and sublevels within an atom. These arrangements dictate an element's chemical reactivity, its bonding behavior, and its physical properties. It follows the Aufbau principle, which dictates that electrons fill the lowest energy levels first, and Hund's rule, which states that electrons will individually occupy each orbital within a subshell before doubling up. The Pauli exclusion principle further limits each orbital to a maximum of two electrons with opposite spins.
Electrons occupy specific orbitals designated by principal quantum numbers (n), which represent energy levels (n=1, 2, 3, etc.), and azimuthal quantum numbers (l), which represent sublevels (s, p, d, f). Each sublevel can hold a specific number of electrons: s (2 electrons), p (6 electrons), d (10 electrons), and f (14 electrons).
Determining Germanium's Electron Configuration
Germanium (Ge) has an atomic number of 32, meaning it possesses 32 protons and, in its neutral state, 32 electrons. To determine its electron configuration, we systematically fill the electron orbitals according to the Aufbau principle and Hund's rule.
The electron configuration of germanium is typically written as: 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p².
Let's break this down:
- 1s²: Two electrons occupy the lowest energy level (n=1), in the s sublevel.
- 2s²: Two electrons occupy the s sublevel of the second energy level (n=2).
- 2p⁶: Six electrons fill the p sublevel of the second energy level.
- 3s²: Two electrons occupy the s sublevel of the third energy level (n=3).
- 3p⁶: Six electrons fill the p sublevel of the third energy level.
- 4s²: Two electrons occupy the s sublevel of the fourth energy level (n=4).
- 3d¹⁰: Ten electrons fill the d sublevel of the third energy level. Note that the 3d sublevel, although having a higher azimuthal quantum number than the 4s, actually has a slightly higher energy level. This is a crucial detail in understanding the filling order.
- 4p²: Two electrons occupy the p sublevel of the fourth energy level.
This configuration indicates that germanium has four valence electrons, located in the 4s and 4p orbitals. These valence electrons are crucial for determining germanium's chemical reactivity and bonding characteristics.
Orbital Diagram of Germanium
A visual representation, using an orbital diagram, can further clarify the electron configuration. Each box represents an orbital, and arrows represent electrons, with opposing arrows indicating paired electrons with opposite spins.
1s: ↑↓
2s: ↑↓
2p: ↑↓ ↑↓ ↑↓
3s: ↑↓
3p: ↑↓ ↑↓ ↑↓
4s: ↑↓
3d: ↑↓ ↑↓ ↑↓ ↑↓ ↑↓
4p: ↑ ↑
This diagram reinforces the presence of two unpaired electrons in the 4p subshell, contributing to germanium's semiconducting properties and its ability to form covalent bonds.
Germanium's Properties and Electron Configuration
Germanium's electron configuration directly influences its key properties:
Semiconducting Behavior
The four valence electrons in germanium are responsible for its semiconducting properties. These electrons can be excited to a higher energy level with relatively low energy input (e.g., heat or light), allowing them to participate in electrical conduction. However, in its pure state, germanium is a relatively poor conductor. Doping with other elements (introducing impurities) can significantly alter its conductivity, creating either n-type (excess electrons) or p-type (electron "holes") semiconductors, essential for electronic devices.
Chemical Bonding
Germanium's four valence electrons enable it to form four covalent bonds. This explains its ability to form tetrahedral structures, crucial in its crystalline form and in compounds such as germanium dioxide (GeO₂). The covalent bonds are relatively strong, contributing to germanium's hardness and high melting point.
Reactivity
While not as reactive as some other elements, germanium can react with halogens (e.g., chlorine, bromine) to form tetrahalides (e.g., GeCl₄), and with oxygen to form germanium dioxide. However, it resists attack by acids and alkalis. Its relatively low reactivity is related to the stability of its filled inner electron shells and the relatively high ionization energies required to remove valence electrons.
Germanium's Applications: A Reflection of its Electron Configuration
The unique properties stemming from germanium's electron configuration make it invaluable in various technological applications:
- Semiconductors: As mentioned, its semiconducting behavior is exploited in transistors, integrated circuits, and other electronic components.
- Fiber Optics: Germanium dioxide is a key component in optical fibers, enabling efficient transmission of light signals over long distances.
- Solar Cells: Germanium's ability to absorb light and convert it into electricity makes it useful in solar cell technology.
- Alloying Agent: Germanium is added to other metals to improve their properties, such as hardness and corrosion resistance.
- Medical Applications: Germanium compounds have found limited use in medical applications, though research is ongoing.
Beyond the Basic Configuration: Excited States and Ions
The electron configuration discussed above represents germanium in its ground state. However, when energy is supplied, electrons can transition to higher energy levels, resulting in excited states. These excited states are temporary and, upon energy dissipation, the electrons return to their ground state configuration.
Similarly, germanium can form ions by losing or gaining electrons. For example, Ge⁴⁺ is a common ion, formed by the loss of all four valence electrons. This results in a completely filled 3d and 3p shell and completely empty 4s and 4p subshells. The configuration of Ge⁴⁺ would then be: 1s²2s²2p⁶3s²3p⁶3d¹⁰. This ion plays a significant role in many of germanium's chemical compounds.
Conclusion: The Significance of Electron Configuration in Understanding Germanium
The electron configuration of germanium, 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p², is not merely a list of numbers and letters. It’s a blueprint for understanding the element's behavior. It explains its semiconducting properties, its chemical bonding characteristics, and its reactivity. By grasping the implications of its electron configuration, we can better appreciate the role germanium plays in numerous technological advancements and its importance across diverse scientific fields. Further research into germanium’s properties and their applications continue to unfold, driven by the fundamental understanding provided by its unique atomic structure. The detailed analysis presented here underscores the central role of electron configuration in shaping the unique properties and technological significance of this remarkable element.
Latest Posts
Latest Posts
-
5 Out Of 6 As A Percent
Apr 08, 2025
-
Does A Trapezoid Have 4 Right Angles
Apr 08, 2025
-
The Central Part Of An Atom Containing Protons And Neutrons
Apr 08, 2025
-
Position The Following Items In Order Of Decreasing Size
Apr 08, 2025
-
Whats The Square Root Of 108
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration Of Germanium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.