What Is The Electron Configuration Of Ca2+
listenit
Mar 19, 2025 · 6 min read
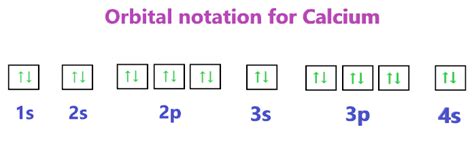
Table of Contents
What is the Electron Configuration of Ca²⁺? A Deep Dive into Calcium's Ionic State
Understanding the electron configuration of ions, particularly those with significant biological and chemical roles, is crucial in chemistry and related fields. Calcium (Ca), a ubiquitous element essential for life, forms a stable divalent cation, Ca²⁺. This article will delve deep into the electron configuration of Ca²⁺, explaining the process of ion formation and its implications. We will explore the relationship between electron configuration and the chemical properties of this crucial ion.
Understanding Electron Configuration
Before we jump into the specifics of Ca²⁺, let's briefly review the concept of electron configuration. The electron configuration of an atom describes the arrangement of electrons in its electron shells and subshells. These arrangements are governed by the principles of quantum mechanics, specifically the Aufbau principle (electrons fill orbitals from lowest to highest energy), Hund's rule (electrons fill orbitals individually before pairing), and the Pauli exclusion principle (no two electrons can have the same four quantum numbers).
These principles dictate that electrons occupy specific orbitals within shells (principal quantum number, n), subshells (azimuthal quantum number, l), and orbitals (magnetic quantum number, ml), with each electron having a unique spin quantum number (ms). Common subshells are represented by s, p, d, and f, capable of holding 2, 6, 10, and 14 electrons, respectively.
Neutral calcium (Ca) has an atomic number of 20, meaning it has 20 protons and, in its neutral state, 20 electrons. Its electron configuration is 1s²2s²2p⁶3s²3p⁶4s². This configuration reflects the filling of orbitals according to the Aufbau principle.
The Formation of Ca²⁺
Calcium readily loses two electrons to achieve a stable octet configuration, resembling the noble gas Argon (Ar). This process is exothermic, meaning it releases energy and makes the ion formation energetically favorable. The loss of electrons occurs from the outermost shell, the 4s orbital, as these electrons are the furthest from the nucleus and experience the least effective nuclear charge.
The formation of the Ca²⁺ ion can be represented as follows:
Ca → Ca²⁺ + 2e⁻
This equation illustrates the loss of two electrons from the neutral calcium atom, resulting in the formation of a positively charged ion, Ca²⁺, and two free electrons.
Electron Configuration of Ca²⁺
Now, the crucial part: what is the electron configuration of the resulting Ca²⁺ ion? Since calcium loses two electrons from its outermost 4s orbital during ionization, the electron configuration of Ca²⁺ becomes 1s²2s²2p⁶3s²3p⁶. This configuration is isoelectronic with Argon, meaning it has the same number of electrons as Argon. The noble gas configuration is exceptionally stable due to the completely filled shells and subshells, contributing to the stability of the Ca²⁺ ion.
This stable configuration makes Ca²⁺ relatively unreactive compared to the neutral calcium atom. Its ionic nature allows for strong electrostatic interactions, forming ionic bonds with anions such as chloride (Cl⁻) or phosphate (PO₄³⁻), which are essential for various biological processes.
Significance of Ca²⁺'s Electron Configuration
The stable electron configuration of Ca²⁺ has profound implications in various fields:
Biological Significance:
- Bone Structure: Calcium ions are vital components of bones and teeth, where they interact with phosphate ions to form the strong and stable hydroxyapatite crystals. The stable ionic nature of Ca²⁺ allows for this crucial structural role.
- Muscle Contraction: Calcium ions are essential for muscle contraction. The influx of Ca²⁺ ions triggers a cascade of events leading to the interaction of actin and myosin filaments, resulting in muscle contraction. The ionic charge and stability of Ca²⁺ are crucial in this process.
- Nerve Impulse Transmission: Ca²⁺ plays a crucial role in neurotransmission, influencing the release of neurotransmitters at synapses. Its electron configuration, leading to its ionic stability, is fundamental to this role.
- Blood Clotting: Calcium ions are essential cofactors in the blood clotting cascade, facilitating the activation and interaction of various clotting factors.
Chemical Significance:
- Ionic Compounds: The divalent nature of Ca²⁺ allows it to form a wide range of stable ionic compounds with various anions. These compounds exhibit different properties depending on the counterion involved, but the stable electron configuration of Ca²⁺ is always a key factor determining the overall stability and behavior of the compound.
- Chemical Reactions: While less reactive than neutral calcium, Ca²⁺ still participates in various chemical reactions, particularly those involving ligand exchange or complex formation. Understanding its electron configuration provides insights into its reactivity and interaction with other molecules or ions.
- Industrial Applications: Calcium compounds, often involving Ca²⁺, have numerous industrial applications, from cement production (calcium silicates) to water treatment (calcium sulfate).
Comparing Ca and Ca²⁺
It's essential to understand the differences between the neutral calcium atom and the Ca²⁺ ion:
| Feature | Ca (Neutral Atom) | Ca²⁺ (Ion) |
|---|---|---|
| Number of electrons | 20 | 18 |
| Electron Configuration | 1s²2s²2p⁶3s²3p⁶4s² | 1s²2s²2p⁶3s²3p⁶ |
| Size | Larger | Smaller |
| Reactivity | More reactive | Less reactive |
| Charge | Neutral (0) | Positive (+2) |
| Stability | Less stable | More stable (isoelectronic with Ar) |
The significant difference in size (Ca²⁺ is smaller than Ca) stems from the increased effective nuclear charge experienced by the remaining electrons after the removal of two electrons from the 4s orbital. This stronger attraction pulls the electrons closer to the nucleus, resulting in a smaller ionic radius. The decrease in size and the gain in stability are key consequences of the ionisation process.
Further Exploration
The electron configuration of Ca²⁺ is not just a theoretical concept; it's a fundamental aspect underlying its widespread importance in biology, chemistry, and industry. Further research can delve into:
- The detailed quantum mechanical calculations that support the electronic structure and the energy levels within the ion.
- The role of Ca²⁺ in specific biochemical pathways, exploring how its interactions with proteins and other molecules are crucial for various biological functions.
- The synthesis and characterization of materials containing Ca²⁺ ions, investigating their structural properties and potential applications.
- Computational methods to model the behaviour of Ca²⁺ in complex systems, providing insights into its interactions and dynamics in various environments.
In conclusion, the electron configuration of Ca²⁺, 1s²2s²2p⁶3s²3p⁶, is a direct result of the loss of two 4s electrons from neutral calcium. This stable, isoelectronic-with-argon configuration is the key to understanding the chemical and biological properties of this ubiquitous and essential ion. The stability and ionic nature of Ca²⁺ are fundamental to its critical roles in countless biological processes and numerous chemical and industrial applications.
Latest Posts
Latest Posts
-
27 Is What Percent Of 90
Mar 19, 2025
-
What Is Half Of 1 5 Teaspoons
Mar 19, 2025
-
12 Is 40 Of What Number
Mar 19, 2025
-
Where Is Rna Found In Cell
Mar 19, 2025
-
What Is 6 Percent Of 15
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration Of Ca2+ . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
