What Is The Electron Configuration Of Ar
listenit
Mar 20, 2025 · 6 min read
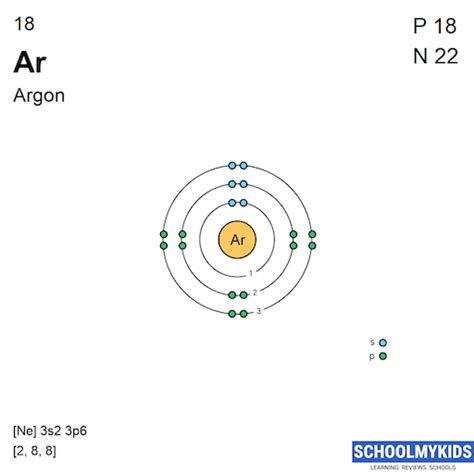
Table of Contents
What is the Electron Configuration of Argon? A Deep Dive into Atomic Structure
Argon (Ar), a noble gas residing in Group 18 of the periodic table, holds a significant place in chemistry and physics due to its unique electronic structure. Understanding its electron configuration is key to comprehending its inertness, its applications, and its role in broader scientific principles. This article will delve into the electron configuration of Argon, explaining its derivation, its significance, and its implications for understanding atomic behavior.
Understanding Electron Configuration
Before exploring Argon's specific electron configuration, let's establish a foundational understanding of what electron configuration represents. An electron configuration is a symbolic notation that describes the arrangement of electrons within the different energy levels and sublevels of an atom. It dictates an atom's chemical properties, reactivity, and how it interacts with other atoms. This arrangement follows specific rules governed by quantum mechanics.
The Aufbau Principle
The Aufbau principle, often translated as the "building-up principle," is a cornerstone of understanding electron configuration. It states that electrons first fill the lowest energy levels available before occupying higher energy levels. This principle dictates the order in which orbitals are filled.
Hund's Rule
Another critical rule is Hund's rule of maximum multiplicity. This rule states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This minimizes electron-electron repulsion and results in a more stable configuration.
Pauli Exclusion Principle
The Pauli exclusion principle is fundamental to understanding electron configurations. It states that no two electrons in an atom can have the same set of four quantum numbers. This means that each orbital can hold a maximum of two electrons, and these two electrons must have opposite spins (represented as +1/2 and -1/2).
Deriving Argon's Electron Configuration
Argon's atomic number is 18, meaning it has 18 protons and, in its neutral state, 18 electrons. To determine its electron configuration, we systematically fill the orbitals according to the Aufbau principle, Hund's rule, and the Pauli exclusion principle.
The order of filling orbitals is generally: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p, and so on.
Let's apply this to Argon:
-
1s²: The first energy level (n=1) contains the 1s subshell, which can hold a maximum of two electrons. These two electrons fill the 1s orbital.
-
2s²: The second energy level (n=2) begins with the 2s subshell, which also holds two electrons.
-
2p⁶: The 2p subshell has three orbitals, each capable of holding two electrons. Therefore, it can hold a total of six electrons.
-
3s²: The 3s subshell, like the 2s, holds two electrons.
-
3p⁶: The 3p subshell, similar to the 2p, also holds six electrons.
Adding the electrons from each subshell, we get a total of 18 electrons (2 + 2 + 6 + 2 + 6 = 18). This completes Argon's electron configuration.
Therefore, the complete electron configuration of Argon is: 1s²2s²2p⁶3s²3p⁶.
Noble Gas Configuration and Argon's Inertness
Argon's electron configuration is particularly significant because it represents a stable octet in its outermost shell (the 3rd shell). The 3s and 3p subshells are completely filled with eight electrons. This stable configuration is characteristic of noble gases and is the primary reason for Argon's inertness.
Noble gases, like Argon, are exceptionally unreactive because their outermost electron shell is completely filled. This means they have little tendency to gain, lose, or share electrons to form chemical bonds. This stability is a direct consequence of their electron configuration. Achieving a stable octet (or a full outermost shell) is a driving force in chemical bonding for many other elements.
Orbital Diagrams and Argon
While the electron configuration notation provides a concise summary, a more detailed representation can be achieved using orbital diagrams. These diagrams visually represent the electrons within each orbital, including their spin. For Argon:
- 1s: ↑↓
- 2s: ↑↓
- 2p: ↑↓ ↑↓ ↑↓
- 3s: ↑↓
- 3p: ↑↓ ↑↓ ↑↓
Each arrow represents an electron, and the direction of the arrow indicates its spin (+1/2 or -1/2). Note that each orbital is filled before pairing electrons according to Hund's rule.
Applications of Argon
Argon's inertness makes it valuable in various applications:
1. Inert Atmosphere for Welding
Argon's unreactive nature makes it ideal for creating an inert atmosphere during welding processes. This prevents oxidation and contamination of the weld, resulting in a stronger and cleaner joint.
2. Light Bulbs and Fluorescent Tubes
Argon is used in some light bulbs and fluorescent tubes to prevent the filament from oxidizing and prolong its lifespan. Its presence contributes to the efficiency and longevity of these light sources.
3. Preservation of Reactive Materials
Argon's inertness provides an excellent environment for storing and handling reactive materials that would otherwise degrade in the presence of air or moisture.
4. Winemaking
In certain winemaking processes, Argon is used to displace oxygen and prevent oxidation during bottling and storage. This helps maintain the quality and flavor profile of the wine.
5. Scientific Instrumentation
Argon finds applications in scientific instruments, such as gas chromatography, where its inertness is crucial for accurate and reliable measurements.
Argon's Isotopes and Electron Configuration
While the most abundant isotope of Argon is Argon-40, other isotopes exist. These isotopes have different numbers of neutrons but the same number of protons and electrons. The electron configuration remains the same regardless of the isotope; only the mass number changes.
Comparing Argon's Electron Configuration to Other Elements
Comparing Argon's electron configuration to other elements helps to understand periodic trends and chemical reactivity. For example, comparing it to Chlorine (1s²2s²2p⁶3s²3p⁵) shows that Chlorine needs one more electron to achieve Argon's stable octet, making it highly reactive. Similarly, Potassium (1s²2s²2p⁶3s²3p⁶4s¹), having one electron more than Argon, readily loses that electron to achieve a stable configuration similar to Argon.
Conclusion
The electron configuration of Argon, 1s²2s²2p⁶3s²3p⁶, is a cornerstone of understanding its unique chemical and physical properties. Its stable octet configuration in the outermost shell is the key to its inertness and its wide range of applications. By understanding the principles of electron configuration – the Aufbau principle, Hund's rule, and the Pauli exclusion principle – we can decipher the behavior of not only Argon but also all elements in the periodic table, providing a deeper understanding of the fundamental building blocks of matter. Further exploration of Argon and its role in various scientific fields will continue to reveal its significance in the world around us.
Latest Posts
Latest Posts
-
64 Is What Percent Of 80
Mar 21, 2025
-
What Is Bigger 3 4 Or 2 3
Mar 21, 2025
-
How Many Oz Is 140 Grams
Mar 21, 2025
-
What Is The Fraction Of 4 5
Mar 21, 2025
-
What Is 33 As A Fraction
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration Of Ar . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
