What Is The Electron Configuration For Tin
listenit
Apr 07, 2025 · 5 min read
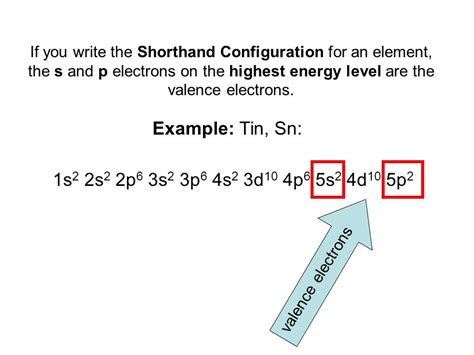
Table of Contents
What is the Electron Configuration for Tin? A Deep Dive into Atomic Structure
Tin (Sn), a post-transition metal with a rich history and diverse applications, presents a fascinating case study in electron configuration. Understanding its electron configuration is crucial to comprehending its chemical properties, reactivity, and the various roles it plays in different fields, from everyday alloys to advanced technologies. This article delves into the electron configuration of tin, exploring its underlying principles and implications.
Understanding Electron Configuration
Before we dive into tin's specific configuration, let's establish a foundational understanding of what electron configuration means. Electron configuration describes the arrangement of electrons in the different energy levels (shells) and sublevels (subshells) within an atom. It follows specific rules dictated by quantum mechanics, most notably the Aufbau principle, the Pauli exclusion principle, and Hund's rule.
- Aufbau principle: Electrons fill the lowest energy levels first. This is like building a house – you start with the foundation before adding the upper floors.
- Pauli exclusion principle: Each orbital can hold a maximum of two electrons, each with opposite spins. Think of an orbital as a room; it can only accommodate two people comfortably, and they need to be different in some way (spin).
- Hund's rule: Electrons fill orbitals within a subshell individually before pairing up. This is like students filling seats in a classroom; they'll occupy individual seats before doubling up.
These principles determine the order in which electrons populate atomic orbitals, resulting in a unique electron configuration for each element.
Determining the Electron Configuration of Tin (Sn)
Tin has an atomic number of 50, meaning it has 50 protons and, in its neutral state, 50 electrons. To determine its electron configuration, we follow the Aufbau principle and fill the orbitals according to their energy levels. The order of filling is typically represented by the following sequence:
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p...
However, understanding this sequence requires acknowledging that energy levels aren't always perfectly separated. There are subtle energy differences influencing the electron filling order, making it sometimes less straightforward.
Following the Aufbau principle and considering the slight energy variations, the electron configuration of tin is:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s² 4d¹⁰ 5p²
Let's break this down:
- 1s²: Two electrons in the first energy level (n=1), in the s subshell.
- 2s² 2p⁶: Eight electrons in the second energy level (n=2), two in the s subshell and six in the p subshell.
- 3s² 3p⁶: Eight electrons in the third energy level (n=3), two in the s subshell and six in the p subshell.
- 4s² 3d¹⁰ 4p⁶: Eighteen electrons in the fourth energy level (n=4); two in the s subshell, ten in the d subshell, and six in the p subshell. Note the 3d subshell filling after 4s.
- 5s² 4d¹⁰ 5p²: Fourteen electrons in the fifth energy level (n=5); two in the s subshell, ten in the d subshell, and two in the p subshell. Again, notice the 4d subshell filling before 5p.
This configuration highlights the presence of two electrons in the outermost (valence) shell, which significantly influences tin's chemical behavior.
Noble Gas Configuration and its Significance
To simplify the electron configuration, we can use the noble gas configuration. Noble gases are elements with completely filled valence shells, resulting in exceptional stability. The noble gas preceding tin is krypton (Kr), with an electron configuration of 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶. Therefore, tin's noble gas configuration is:
[Kr] 5s² 4d¹⁰ 5p²
This notation is more concise and emphasizes the electrons beyond the stable krypton core. The remaining electrons, those in the 5s and 5p subshells, are the valence electrons responsible for tin's chemical bonding.
Chemical Properties and Electron Configuration
Tin's electron configuration directly explains its chemical properties. The two valence electrons in the 5s and 5p orbitals readily participate in chemical reactions. Tin can lose these two electrons to form a +2 oxidation state (Sn²⁺) or all four valence electrons to achieve a +4 oxidation state (Sn⁴⁺). The +2 state is more common in compounds where the 5s electrons are involved in bonding, while the +4 state involves the participation of both 5s and 5p electrons. This versatility in oxidation states explains tin's ability to form various compounds.
The existence of two common oxidation states results in tin's ability to form a diverse range of compounds with different elements. For example, tin(II) chloride (SnCl₂) and tin(IV) chloride (SnCl₄) demonstrate the differing reactivities depending on the oxidation state.
Applications and Electron Configuration
The unique electron configuration of tin directly impacts its diverse applications:
- Alloys: Tin's ability to form alloys with various metals, such as copper (bronze) and lead (solder), is crucial to its widespread use in construction, electronics, and plumbing. The electron configuration dictates its bonding capabilities within these alloys.
- Coatings: Tin plating protects other metals from corrosion, leveraging its relative inertness stemming from its electron configuration and resulting stability.
- Organotin Compounds: Organotin compounds, where tin bonds to carbon atoms, have applications as biocides, stabilizers in plastics, and catalysts. The electron configuration is key to understanding the nature of these bonds and the resulting functionality.
- Solder: Tin-lead solder is traditionally used for joining electronic components. The melting point of the alloy is dependent on the electron configuration-driven interactions between tin and lead atoms.
Conclusion: The Importance of Understanding Tin's Electron Configuration
The electron configuration of tin, whether expressed in its full or noble gas notation, is fundamental to understanding its behavior and applications. Its two valence electrons and the ability to achieve two stable oxidation states (+2 and +4) influence its reactivity and the diverse range of compounds and alloys it can form. From the Bronze Age to modern electronics, tin's unique atomic structure, embodied by its electron configuration, underscores its importance in human civilization and technological advancement.
Further exploration of tin's chemistry, including its reactions and compound formation, can only be fully appreciated with a thorough grasp of its electron configuration and the underlying principles of atomic structure. This knowledge provides the key to unlocking the secrets behind this versatile and historically significant element.
Latest Posts
Latest Posts
-
How Many Valence Electrons In Ne
Apr 08, 2025
-
Why Do Bones Heal Quicker Than Cartilage
Apr 08, 2025
-
What Is The Area Of Triangle Rst
Apr 08, 2025
-
New Ocean Crust Is Formed At
Apr 08, 2025
-
Why Is The Melting Of Ice Not A Chemical Reaction
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration For Tin . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.