What Is The Electron Configuration For Phosphorus
listenit
Mar 15, 2025 · 6 min read
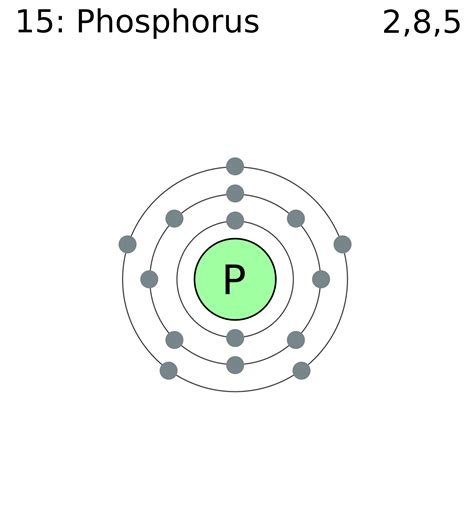
Table of Contents
What is the Electron Configuration for Phosphorus? A Deep Dive into Atomic Structure
Phosphorus, a vital element for life, boasts a fascinating electron configuration that dictates its chemical properties and reactivity. Understanding this configuration is key to comprehending its role in biological systems, industrial processes, and even the creation of novel materials. This in-depth exploration will delve into the electron configuration of phosphorus, examining its underlying principles, exceptions, and implications.
Understanding Electron Configuration
Before we dive into phosphorus specifically, let's establish a fundamental understanding of electron configuration. An electron configuration describes the arrangement of electrons in the electron shells and subshells of an atom. It's essentially an address for each electron, specifying its energy level and orbital type. This arrangement follows specific rules governed by quantum mechanics:
-
Aufbau Principle: Electrons fill orbitals starting from the lowest energy levels and progressing upwards. Think of it like filling a building from the ground floor to the top.
-
Pauli Exclusion Principle: Each orbital can hold a maximum of two electrons, each with opposite spin (represented as ↑ and ↓). It's like a room that can only accommodate two people.
-
Hund's Rule: Within a subshell, electrons will individually occupy each orbital before doubling up in any single orbital. This minimizes electron-electron repulsion. Imagine individuals preferring to have their own rooms before sharing.
These principles are crucial in determining the order in which electrons fill orbitals, resulting in a unique electron configuration for each element.
Determining the Electron Configuration of Phosphorus
Phosphorus (P) has an atomic number of 15, meaning it possesses 15 protons and, in a neutral atom, 15 electrons. To determine its electron configuration, we follow the Aufbau principle and fill the orbitals accordingly:
1s², 2s², 2p⁶, 3s², 3p³
Let's break this down:
-
1s²: The first energy level (n=1) contains one subshell, the 's' subshell, which can hold up to two electrons. Both are filled.
-
2s²: The second energy level (n=2) also has an 's' subshell, which is again filled with two electrons.
-
2p⁶: The second energy level also contains a 'p' subshell, which can hold up to six electrons (three orbitals, each holding two). This subshell is completely filled.
-
3s²: The third energy level (n=3) begins with an 's' subshell containing two electrons.
-
3p³: Finally, the third energy level's 'p' subshell contains three electrons. Crucially, these three electrons occupy separate orbitals within the 'p' subshell, following Hund's Rule. This leaves one orbital empty.
Therefore, the full electron configuration for phosphorus is 1s² 2s² 2p⁶ 3s² 3p³. This configuration directly impacts phosphorus's chemical behavior.
The Significance of the 3p³ Subshell
The partially filled 3p subshell is the key to understanding phosphorus's reactivity. With three unpaired electrons, phosphorus readily forms covalent bonds with other atoms to achieve a more stable electron configuration. It often forms three bonds, striving to complete its octet (eight electrons in its valence shell).
This tendency to form three bonds explains phosphorus's presence in numerous compounds. For instance, in phosphine (PH₃), phosphorus shares three electrons with three hydrogen atoms, achieving a stable octet. Similarly, in phosphorus trichloride (PCl₃), it bonds with three chlorine atoms.
Phosphorus's Various Oxidation States
The partially filled 3p subshell also allows phosphorus to exhibit a variety of oxidation states. While it commonly displays an oxidation state of +3 (as seen in PCl₃), it can also achieve oxidation states of +5 (as in PCl₅) and even negative oxidation states (-3) in compounds like phosphides. This versatility contributes to its extensive applications.
+3 Oxidation State:
This oxidation state arises from the participation of the three 3p electrons in bonding. The resulting compounds, like phosphorus trichloride, are often reactive and easily oxidized.
+5 Oxidation State:
Achieving a +5 oxidation state involves the participation of the 3s and all three 3p electrons. This results in compounds like phosphorus pentachloride, which demonstrates stronger oxidizing power compared to +3 oxidation state compounds. The +5 oxidation state is particularly relevant in phosphate ions, crucial for biological systems.
Negative Oxidation State (-3):
In this state, phosphorus gains three electrons to achieve a stable octet, commonly forming ionic compounds with metals, known as phosphides.
Orbital Diagrams and Electron Configuration
A visual representation of the electron configuration is crucial for a complete understanding. Orbital diagrams illustrate the arrangement of electrons within each subshell, including the spin of each electron. For phosphorus, the orbital diagram would show:
- 1s: ↑↑
- 2s: ↑↑
- 2p: ↑↑ ↑↑ ↑↑
- 3s: ↑↑
- 3p: ↑ ↑ ↑
This diagram visually confirms the three unpaired electrons in the 3p subshell, explaining phosphorus's tendency to form three covalent bonds.
Applications Based on Electron Configuration
The unique electron configuration of phosphorus directly influences its diverse applications across numerous fields:
-
Fertilizers: Phosphorus is an essential nutrient for plant growth, and its compounds are extensively used in fertilizers to enhance crop yields. The availability of phosphorus, in forms like phosphates, directly impacts global food security.
-
Matches: The ease with which phosphorus reacts with oxygen makes it a key component in match heads. The reaction generates the heat and light necessary to ignite the match.
-
Detergents: Phosphates were formerly widely used in detergents for their water-softening properties. However, their impact on aquatic ecosystems has led to a decline in their use.
-
Semiconductors: Phosphorus is a crucial dopant in semiconductor materials, modifying their electrical conductivity. This property is essential for the electronics industry.
-
Biological Systems: Phosphorus plays a crucial role in various biological processes, including energy transfer (ATP), DNA structure, and cell signaling. The unique reactivity enabled by its electron configuration underpins these functions.
Exceptions and Complexities
While the Aufbau principle provides a general framework for predicting electron configurations, some exceptions exist. These exceptions often involve partially filled or nearly filled subshells, where electron-electron repulsions can influence orbital filling. Phosphorus, however, adheres to the Aufbau principle without exception.
Conclusion
The electron configuration of phosphorus, 1s² 2s² 2p⁶ 3s² 3p³, is far more than just a set of numbers. It represents the fundamental building block of its chemical behavior and its diverse applications. The partially filled 3p subshell, with its three unpaired electrons, directly governs phosphorus's reactivity, allowing it to form stable bonds and exhibit multiple oxidation states. This understanding is critical for appreciating phosphorus's importance in agriculture, industry, and the life sciences. From fertilizers to semiconductors to the intricate workings of biological systems, phosphorus's unique electron configuration has far-reaching consequences. Further exploration into the intricacies of electron configurations, orbital interactions, and chemical bonding can reveal deeper insights into the diverse roles of this essential element.
Latest Posts
Latest Posts
-
3 1 3 Divided By 4
Mar 15, 2025
-
Why Does Acceleration Of A Car Decrease When Speed Increases
Mar 15, 2025
-
Solving Absolute Value Equations On Both Sides
Mar 15, 2025
-
Solving Systems Of Equations With 3 Variables
Mar 15, 2025
-
What Is 1 875 As A Fraction
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration For Phosphorus . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
