What Is The Electron Configuration For P
listenit
Mar 16, 2025 · 6 min read
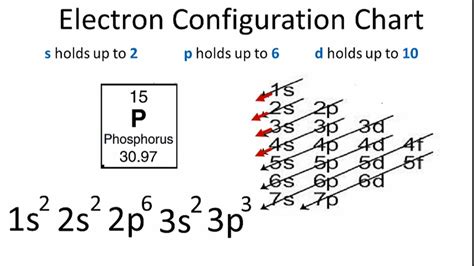
Table of Contents
What is the Electron Configuration for P (Phosphorus)? A Deep Dive into Atomic Structure
Phosphorus, a nonmetal element crucial for life, presents a fascinating case study in understanding electron configuration. This article will delve into the electron configuration of phosphorus (P), explaining its significance and exploring the underlying principles of atomic structure that govern it. We will also explore how this configuration dictates its chemical properties and reactivity.
Understanding Electron Configuration
Electron configuration describes the arrangement of electrons within the electron shells and subshells of an atom. It follows specific rules dictated by quantum mechanics, which governs the behavior of electrons at the atomic level. Understanding electron configuration is fundamental to comprehending an element's chemical behavior, bonding preferences, and reactivity.
The Aufbau Principle and Hund's Rule
Two crucial principles guide the filling of electrons into orbitals:
-
The Aufbau Principle: This principle states that electrons first fill the lowest energy levels available before moving to higher energy levels. This is like filling a building from the ground floor upwards – you don't start on the top floor!
-
Hund's Rule: This rule dictates that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. Think of it as electrons preferring to be alone in their own rooms before sharing. They want to minimize repulsion.
Orbitals and Subshells
Electrons reside in orbitals, which are regions of space around the nucleus where there's a high probability of finding an electron. These orbitals are grouped into subshells (s, p, d, f), each with a specific number of orbitals and a characteristic energy level.
- s subshell: Contains one orbital, holding a maximum of two electrons.
- p subshell: Contains three orbitals, holding a maximum of six electrons.
- d subshell: Contains five orbitals, holding a maximum of ten electrons.
- f subshell: Contains seven orbitals, holding a maximum of fourteen electrons.
Determining the Electron Configuration of Phosphorus (P)
Phosphorus (P) has an atomic number of 15, meaning it has 15 protons and 15 electrons in a neutral atom. To determine its electron configuration, we follow the Aufbau principle and Hund's rule:
-
First Shell (n=1): This shell contains only the 1s subshell, which can hold two electrons. So we fill it completely: 1s².
-
Second Shell (n=2): This shell contains the 2s and 2p subshells. The 2s subshell is filled first with two electrons: 2s². The 2p subshell has three orbitals, each holding a maximum of two electrons, for a total of six electrons. We fill it with six electrons: 2p⁶.
-
Third Shell (n=3): This shell contains the 3s and 3p subshells. The 3s subshell holds two electrons: 3s². Phosphorus has 15 electrons, and we've accounted for 10 (2 + 2 + 6). This leaves 5 electrons to fill the 3p subshell. According to Hund's rule, these five electrons will occupy each of the three 3p orbitals individually before pairing up. This results in 3p³.
Therefore, the complete electron configuration for phosphorus is: 1s²2s²2p⁶3s²3p³
This can also be written in a condensed notation, using the noble gas configuration for the preceding noble gas (Neon, Ne): [Ne] 3s²3p³
Significance of Phosphorus's Electron Configuration
The electron configuration of phosphorus directly impacts its chemical properties and behavior:
-
Valence Electrons: The outermost electrons (3s²3p³) are the valence electrons. These electrons are involved in chemical bonding, determining the element's reactivity. Phosphorus has five valence electrons, meaning it can readily form three covalent bonds to achieve a stable octet (eight electrons in its outermost shell), as seen in molecules like PCl₃. It can also form five bonds in situations like PCl₅, utilizing the d orbitals from the next energy level to accommodate more bonds (expanded octet).
-
Reactivity: The presence of three unpaired electrons in the 3p subshell makes phosphorus highly reactive. It readily forms covalent bonds with other elements, especially those that can contribute electrons to complete its octet. This reactivity is crucial for its role in biological systems.
-
Allotropes: Phosphorus exhibits allotropy, existing in different structural forms with varying properties. This is partly due to the way its valence electrons can participate in bonding, forming different arrangements of atoms. White phosphorus, for example, is highly reactive due to the arrangement of its atoms. Red phosphorus is less reactive due to a different molecular structure.
-
Oxidation States: The five valence electrons allow phosphorus to exhibit a variety of oxidation states, from -3 (as in phosphides) to +5 (as in phosphates). This versatility contributes to its diverse chemistry and its importance in various compounds.
Phosphorus in Biological Systems
The electron configuration of phosphorus is intrinsically linked to its essential role in biological systems. Its reactivity and ability to form stable bonds make it crucial for:
-
DNA and RNA: Phosphorus is a fundamental component of the backbone of DNA and RNA molecules. The phosphate groups link the sugar molecules in these nucleic acids, enabling the storage and transfer of genetic information.
-
ATP (Adenosine Triphosphate): ATP, the energy currency of cells, contains phosphate groups. The hydrolysis (breaking) of phosphate bonds in ATP releases energy that drives numerous cellular processes.
-
Bones and Teeth: Phosphorus is a major component of bones and teeth, where it contributes to their strength and structural integrity in the form of calcium phosphate.
Applications of Phosphorus and its Compounds
The unique properties stemming from its electron configuration lead to the widespread application of phosphorus and its compounds in various industries:
-
Fertilizers: Phosphorus is a crucial nutrient for plant growth, and phosphate-containing fertilizers are essential for boosting agricultural yields.
-
Detergents: Phosphates were once commonly used in detergents, but their use is now more restricted due to environmental concerns.
-
Matches: Red phosphorus is used in the striking surface of matchboxes.
-
Flame Retardants: Organophosphorus compounds are effective flame retardants, providing fire protection in various materials.
-
Semiconductors: Phosphorus is used in the production of semiconductors, exploiting its ability to introduce controlled electron deficiencies.
Further Exploration: Beyond the Basics
The electron configuration of phosphorus provides a foundation for understanding its behavior. However, a more in-depth analysis necessitates considering other factors:
-
Orbital Hybridization: In some compounds, phosphorus's atomic orbitals hybridize, forming new orbitals with different energy levels and orientations. This hybridization influences the molecular geometry and bonding characteristics.
-
Molecular Orbital Theory: This theory provides a more sophisticated description of bonding, considering the interaction of atomic orbitals to form molecular orbitals. This approach offers deeper insights into bond strength and reactivity.
Conclusion
The seemingly simple electron configuration of phosphorus (1s²2s²2p⁶3s²3p³) holds the key to understanding its remarkable properties and its indispensable role in the natural world. From the genetic information encoded in DNA to the energy powering our cells, phosphorus's unique electronic structure shapes our world in profound ways. This configuration, governed by fundamental principles of quantum mechanics, provides a lens through which we can appreciate the complexity and elegance of chemistry at the atomic level. Further investigation into its behavior beyond the basic electron configuration opens doors to a richer understanding of its multifaceted chemistry and its vital significance across numerous scientific disciplines.
Latest Posts
Latest Posts
-
The Elbow Is Proximal To The Shoulder
Mar 17, 2025
-
How Many Radians In A Revolution
Mar 17, 2025
-
How Can Sedimentary Rock Become Metamorphic Rock
Mar 17, 2025
-
What Is The Square Root Of 500
Mar 17, 2025
-
What Is The Next Number In The Sequence 3 9 27 81
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration For P . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
