What Is The Electron Configuration For Ga
listenit
Mar 17, 2025 · 7 min read
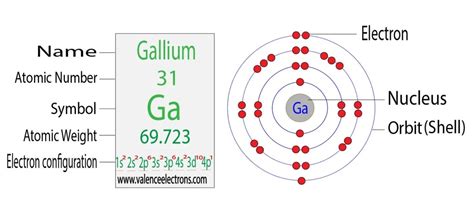
Table of Contents
What is the Electron Configuration for Ga? A Deep Dive into Gallium's Atomic Structure
Gallium (Ga), a fascinating element with a melting point lower than body temperature, finds extensive use in semiconductors, LEDs, and various other applications. Understanding its electronic structure, specifically its electron configuration, is crucial to comprehending its properties and behavior. This article provides a comprehensive exploration of gallium's electron configuration, delving into the underlying principles and its implications.
Understanding Electron Configuration
Before diving into gallium's specific configuration, let's establish a foundational understanding of what electron configuration represents. Electron configuration describes the arrangement of electrons in the different energy levels and sublevels within an atom. It follows the Aufbau principle, which dictates that electrons fill the lowest energy levels first before moving to higher ones. The Pauli exclusion principle states that no two electrons in an atom can have the same set of four quantum numbers (n, l, ml, and ms), meaning each orbital can hold a maximum of two electrons with opposite spins. Finally, Hund's rule specifies that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital.
These principles help predict the electron configuration of any element, providing a blueprint of its atomic structure and, consequently, its chemical properties. The configuration is often expressed using a shorthand notation indicating the principal energy level (n), the subshell (s, p, d, or f), and the number of electrons in that subshell.
Determining the Electron Configuration of Gallium (Ga)
Gallium's atomic number is 31, meaning it has 31 protons and, in its neutral state, 31 electrons. To determine its electron configuration, we follow the Aufbau principle, filling orbitals in order of increasing energy:
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p...
Each subshell has a specific capacity:
- s subshell: Holds a maximum of 2 electrons.
- p subshell: Holds a maximum of 6 electrons.
- d subshell: Holds a maximum of 10 electrons.
- f subshell: Holds a maximum of 14 electrons.
Following this order, we can fill the orbitals for gallium's 31 electrons:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p¹
This is the complete electron configuration for gallium. Note that the 4s subshell fills before the 3d subshell, even though the 3d subshell is at a lower principal quantum number (n=3). This is because the 4s subshell has a slightly lower energy level than the 3d subshell. Similarly, the 4p subshell is filled after the 3d subshell, despite having a higher principal quantum number.
We can also express this configuration in a more concise noble gas configuration. We find the nearest noble gas with a lower atomic number than gallium, which is Argon (Ar) with atomic number 18. Argon's electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁶. Therefore, gallium's noble gas configuration is:
[Ar] 4s² 3d¹⁰ 4p¹
This notation simplifies the representation while still conveying all the necessary information.
Understanding the Implications of Gallium's Electron Configuration
Gallium's electron configuration dictates many of its chemical and physical properties. The presence of a single electron in the 4p subshell explains why gallium readily loses one electron to form a +1 oxidation state. However, it can also lose three electrons to achieve a +3 oxidation state, a feature commonly observed in its compounds. This ability to exhibit multiple oxidation states contributes to gallium's versatility in chemical reactions and its wide range of applications.
The filled 3d subshell contributes to gallium's relatively high density compared to other elements in its period. The filled d orbitals also shield the outer electrons, influencing its atomic radius and reactivity.
Gallium's Chemical Properties and its Electron Configuration
Gallium's chemical behavior is directly linked to its electron configuration. The outermost electron in the 4p orbital is relatively loosely held, making it relatively easy to lose in chemical reactions. This explains its tendency to form compounds where it exhibits a +3 oxidation state, losing three electrons from the 4s and 4p orbitals. This +3 oxidation state is its most stable and common state. However, the availability of the 4s electrons makes it possible to form +1 oxidation states under certain conditions, leading to a range of compounds. This versatility in oxidation states distinguishes gallium's chemistry and provides a basis for the diverse array of compounds it forms.
Gallium's Physical Properties and its Electron Configuration
Gallium's electron configuration also significantly contributes to its physical properties. The electron distribution within its shells and subshells influences its metallic bonding, influencing attributes such as melting point, electrical conductivity, and thermal conductivity. The comparatively weak attraction between its valence electrons and the nucleus explains its relatively low melting point. Its metallic bonding, owing to the valence electrons in the 4s and 4p orbitals, contributes to its excellent electrical and thermal conductivity, essential characteristics for its use in semiconductor devices.
Gallium's Applications and the Significance of its Electron Configuration
The unique properties stemming from gallium's electron configuration have led to its widespread use in numerous technologies.
-
Semiconductors: Gallium arsenide (GaAs) and other gallium-containing compounds are crucial in semiconductor technology, particularly in high-frequency and high-power applications, owing to their superior electron mobility and energy bandgaps. The ability of gallium to form stable bonds with other elements, including arsenic, is directly linked to its electron configuration.
-
LEDs (Light Emitting Diodes): Gallium nitride (GaN) is a key component in high-brightness LEDs, exhibiting excellent efficiency in converting electrical energy into light. The electronic structure of gallium and nitrogen allows for efficient radiative recombination of electrons and holes, producing light.
-
Solar Cells: Gallium arsenide (GaAs) and related materials are employed in high-efficiency solar cells, showcasing better performance than silicon-based alternatives. Again, this superior performance can be attributed to its electron configuration, leading to the efficient absorption of sunlight.
-
Medical Applications: Gallium isotopes are used in medical imaging and cancer treatments. Their properties stem from their nuclear structure, but the chemical properties which govern the body's interaction with them are deeply rooted in the electronic configuration.
-
Other Applications: Gallium finds use in various other applications, including alloying agents in low-melting-point alloys, high-temperature thermometers, and more.
Advanced Concepts and Further Exploration
A deeper understanding of gallium's electron configuration involves exploring concepts such as orbital hybridization, molecular orbital theory, and quantum mechanics. These concepts provide a more nuanced picture of the electron distribution and how it impacts the properties and reactivity of gallium.
Orbital Hybridization
When gallium forms compounds, its atomic orbitals can hybridize to form new hybrid orbitals. This hybridization affects the geometry and bonding characteristics of the molecules. For example, in gallium trichloride (GaCl₃), the valence orbitals of gallium (4s and 4p) hybridize to form sp² hybrid orbitals, resulting in a trigonal planar molecular geometry. This geometry is a direct consequence of the hybridization that arises from gallium's specific electron configuration.
Molecular Orbital Theory
Molecular orbital theory extends the principles of electron configuration to molecules. This theory helps predict the bonding and antibonding orbitals in gallium compounds, offering insights into their stability and reactivity. For instance, understanding the molecular orbitals of gallium arsenide is crucial in designing and optimizing its use in semiconductor devices. The energy levels and occupancy of these orbitals are determined by the electron configurations of the constituent atoms, gallium and arsenic, in this case.
Quantum Mechanics and Electron Configuration
The electron configuration itself is a consequence of the principles of quantum mechanics. The quantum numbers describing each electron (principal quantum number (n), azimuthal quantum number (l), magnetic quantum number (ml), and spin quantum number (ms)) determine the orbital's energy, shape, and orientation, and ultimately, the electron configuration. A deeper study of quantum mechanics is necessary for a truly thorough understanding of the underlying forces governing the electron arrangement in gallium and other elements.
Conclusion
In conclusion, the electron configuration of gallium ([Ar] 4s² 3d¹⁰ 4p¹) is fundamental to understanding its properties, reactivity, and diverse applications. This configuration dictates its ability to form multiple oxidation states, influencing its chemistry. It also underlies its physical properties, particularly its low melting point and its excellent electrical and thermal conductivities, essential features determining its utility in semiconductors, LEDs, and various other technologies. Further exploration of advanced concepts like orbital hybridization and molecular orbital theory adds layers of complexity and enhances our comprehensive understanding of this important element. The significance of gallium's electron configuration extends beyond simple academic interest and is integral to its practical application in various fields of science and technology.
Latest Posts
Latest Posts
-
Least Common Multiple Of 2 And 8
Mar 17, 2025
-
Evaluate The Integral By Interpreting It In Terms Of Areas
Mar 17, 2025
-
1 1 4 Divided By 3
Mar 17, 2025
-
Can Crayfish Live Out Of Water
Mar 17, 2025
-
Domain Of 1 X 2 1
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration For Ga . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
