What Is The Difference Between An Atom And A Ion
listenit
Mar 18, 2025 · 6 min read
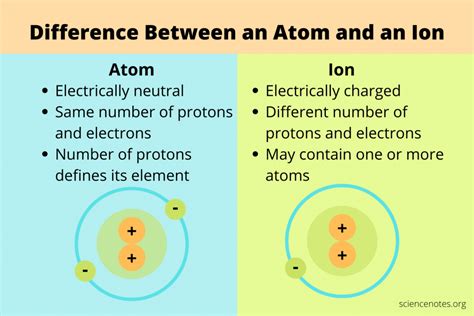
Table of Contents
What's the Difference Between an Atom and an Ion? A Deep Dive into Atomic Structure and Charge
Understanding the fundamental building blocks of matter is crucial to grasping the complexities of chemistry and physics. At the heart of this understanding lies the distinction between an atom and an ion. While both are incredibly small particles, a subtle yet significant difference in their structure defines their distinct properties and behaviors. This article will delve deep into the intricacies of atoms and ions, exploring their fundamental characteristics, the processes that lead to ion formation, and the crucial roles they play in various scientific disciplines.
Atoms: The Fundamental Building Blocks
An atom is the smallest unit of an element that retains the chemical properties of that element. Think of it as the basic, uncharged Lego brick of all matter. It's composed of three primary subatomic particles:
1. Protons: Positively Charged Core
Protons reside in the atom's nucleus, the dense central region. They carry a positive electrical charge (+1) and contribute significantly to the atom's mass. The number of protons in an atom's nucleus defines its atomic number and uniquely identifies the element. For example, an atom with one proton is hydrogen, an atom with six protons is carbon, and an atom with 92 protons is uranium.
2. Neutrons: Neutral Nuclear Partners
Neutrons, also located within the nucleus, are electrically neutral, possessing no charge. They contribute to the atom's mass but don't directly influence its chemical properties. The number of neutrons in an atom can vary, leading to isotopes of the same element (atoms with the same number of protons but different numbers of neutrons). For instance, carbon-12 and carbon-14 are isotopes of carbon, with 6 and 8 neutrons respectively.
3. Electrons: Negatively Charged Orbitals
Electrons are much smaller and lighter than protons and neutrons and orbit the nucleus in specific energy levels or shells. They carry a negative electrical charge (-1). The arrangement of electrons in these shells determines the atom's chemical behavior and how it interacts with other atoms. The outermost shell, known as the valence shell, plays a particularly crucial role in chemical bonding. The number of electrons in a neutral atom is equal to the number of protons, ensuring a balanced, overall neutral charge.
Ions: Charged Atoms
An ion is an atom or molecule that has gained or lost one or more electrons, resulting in a net electrical charge. This charge imbalance is what fundamentally distinguishes an ion from a neutral atom.
1. Cations: Positively Charged Ions
A cation is a positively charged ion, formed when an atom loses one or more electrons. This loss typically occurs because the atom's valence shell is relatively unstable or only partially filled. By losing electrons, the atom achieves a more stable electron configuration, often resembling a noble gas (a highly stable group of elements). For example, sodium (Na) readily loses one electron to become a sodium cation (Na+), achieving a stable electron configuration like neon.
2. Anions: Negatively Charged Ions
An anion is a negatively charged ion, formed when an atom gains one or more electrons. This gain occurs because the atom's valence shell is almost full. By gaining electrons, the atom completes its valence shell, achieving a more stable configuration. For example, chlorine (Cl) readily gains one electron to become a chloride anion (Cl-), achieving a stable electron configuration like argon.
The Process of Ion Formation: Ionization
The process of converting a neutral atom into an ion is called ionization. This can occur through various mechanisms:
-
Electron Transfer: This is the most common mechanism, involving the direct transfer of electrons between atoms. This often happens during chemical reactions, where atoms with a strong tendency to lose electrons (metals) react with atoms with a strong tendency to gain electrons (non-metals).
-
Electromagnetic Radiation: High-energy electromagnetic radiation, such as X-rays or gamma rays, can knock electrons out of atoms, creating cations. This is the principle behind ionization detectors used in various applications.
-
Collisions: Atoms can also be ionized through collisions with high-energy particles, such as those found in radioactive materials or particle accelerators.
The Importance of Ions: A Crucial Role in Nature and Technology
Ions play a vital role in numerous natural processes and technological applications.
Ions in Biological Systems:
-
Electrolyte Balance: Ions like sodium (Na+), potassium (K+), calcium (Ca2+), and chloride (Cl-) are essential electrolytes in the human body, maintaining proper fluid balance, nerve impulse transmission, and muscle contraction.
-
Enzyme Function: Many enzymes, biological catalysts, require specific ions for their activity.
-
DNA Structure: The negatively charged phosphate backbone of DNA relies on ionic interactions to maintain its double helix structure.
Ions in Chemical Reactions:
-
Ionic Bonding: Ions form ionic bonds, a type of chemical bond formed by electrostatic attraction between oppositely charged ions. This leads to the formation of ionic compounds, such as sodium chloride (NaCl, table salt).
-
Electrochemical Reactions: Ions are essential participants in electrochemical reactions, which form the basis of batteries and fuel cells.
Ions in Technology:
-
Semiconductors: The controlled addition of impurities (doping) to create p-type and n-type semiconductors relies on the introduction of ions. This is crucial in the manufacturing of electronic devices like transistors and integrated circuits.
-
Electroplating: Electroplating involves using ions in an electrolytic solution to deposit a thin layer of metal onto a surface, enhancing its properties.
-
Mass Spectrometry: Mass spectrometry uses ions to analyze the composition of different substances. The technique involves ionizing a sample and then separating the resulting ions based on their mass-to-charge ratio.
Distinguishing Features: A Summary Table
| Feature | Atom | Ion |
|---|---|---|
| Charge | Neutral (0) | Positive (cation) or negative (anion) |
| Electron Count | Equal to proton count | Unequal to proton count |
| Stability | Varies; noble gases are most stable | Generally more stable than the parent atom |
| Formation | Fundamental unit of element | Formed by ionization |
| Chemical Behavior | Determined by valence electrons | Determined by charge and electron configuration |
Conclusion: A Fundamental Distinction with Far-Reaching Implications
The seemingly small difference between an atom and an ion – the presence or absence of a net electrical charge – has enormous consequences in the world around us. Understanding this distinction is key to appreciating the intricacies of chemical reactions, biological processes, and technological advancements. From the electrolyte balance in our bodies to the functioning of electronic devices, ions are central players in a vast array of phenomena. By grasping the fundamental nature of atoms and ions, we can better understand the universe at a molecular level.
Latest Posts
Latest Posts
-
1500 Ml Is How Many Liters
Mar 18, 2025
-
During Which Phase Do Chromosomes First Become Visible
Mar 18, 2025
-
Do You Always Use The Henderson Hasselbalch For Titrations
Mar 18, 2025
-
What Is The Fraction For 20
Mar 18, 2025
-
What Is 80 Percent Of 65
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Is The Difference Between An Atom And A Ion . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
