What Is The Conjugate Base Of H2so4
listenit
Mar 21, 2025 · 5 min read
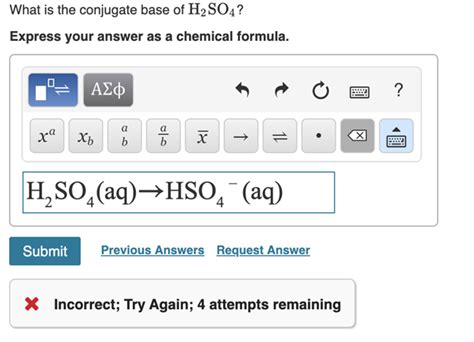
Table of Contents
What is the Conjugate Base of H₂SO₄? A Deep Dive into Acid-Base Chemistry
Sulfuric acid (H₂SO₄), a potent and ubiquitous strong acid, plays a crucial role in numerous industrial processes and chemical reactions. Understanding its properties, particularly its conjugate base, is vital for comprehending its behavior in various chemical contexts. This article will delve into the concept of conjugate bases, explore the conjugate base of H₂SO₄, discuss its properties, and examine its significance in different chemical applications.
Understanding Conjugate Acid-Base Pairs
Before we delve into the specifics of sulfuric acid's conjugate base, let's establish a firm understanding of the fundamental concept of conjugate acid-base pairs. According to Brønsted-Lowry acid-base theory, an acid is a substance that donates a proton (H⁺), while a base is a substance that accepts a proton. When an acid donates a proton, it forms its conjugate base. Conversely, when a base accepts a proton, it forms its conjugate acid.
The relationship between an acid and its conjugate base is characterized by a difference of only one proton (H⁺). They are essentially the same molecule, differing only by the presence or absence of this single proton. For example:
- HCl (acid) + H₂O (base) ⇌ Cl⁻ (conjugate base) + H₃O⁺ (conjugate acid)
In this reaction, hydrochloric acid (HCl) donates a proton to water (H₂O), forming the chloride ion (Cl⁻), its conjugate base, and the hydronium ion (H₃O⁺), the conjugate acid of water.
The Conjugate Base of H₂SO₄: A Step-wise Approach
Sulfuric acid, H₂SO₄, is a diprotic acid, meaning it can donate two protons. This means it has two dissociation steps, each producing a conjugate base. Let's examine these steps individually:
First Dissociation: HSO₄⁻
The first dissociation of sulfuric acid in water is a strong acid dissociation:
H₂SO₄(aq) + H₂O(l) → HSO₄⁻(aq) + H₃O⁺(aq)
In this step, sulfuric acid donates one proton to water, resulting in the formation of the hydrogen sulfate ion (HSO₄⁻) and the hydronium ion (H₃O⁺). Therefore, the first conjugate base of H₂SO₄ is HSO₄⁻. This is a relatively strong acid itself, but significantly weaker than H₂SO₄.
Second Dissociation: SO₄²⁻
The hydrogen sulfate ion (HSO₄⁻) can further donate a proton in a second dissociation step:
HSO₄⁻(aq) + H₂O(l) ⇌ SO₄²⁻(aq) + H₃O⁺(aq)
This second dissociation is significantly weaker than the first. HSO₄⁻ is a weak acid. In this step, the hydrogen sulfate ion donates a proton to water, yielding the sulfate ion (SO₄²⁻) and another hydronium ion. Therefore, the second conjugate base of H₂SO₄ is SO₄²⁻. The sulfate ion is the fully deprotonated form of sulfuric acid and is a very weak base.
Properties of the Conjugate Bases of H₂SO₄
The conjugate bases of sulfuric acid, HSO₄⁻ and SO₄²⁻, exhibit distinct properties that influence their behavior in chemical reactions:
Hydrogen Sulfate Ion (HSO₄⁻):
-
Amphoteric Nature: HSO₄⁻ is amphoteric, meaning it can act as both an acid and a base. As shown above, it can donate a proton to act as an acid. It can also accept a proton, acting as a base: HSO₄⁻(aq) + H⁺(aq) → H₂SO₄(aq)
-
Acid Strength: While a weaker acid than H₂SO₄, HSO₄⁻ is still considered a moderately strong acid. Its dissociation constant (Ka) is significantly larger than that of many weak acids. This means it readily donates its proton to other bases in solution.
-
Solubility: Hydrogen sulfate salts are generally soluble in water, except for a few exceptions, like barium hydrogen sulfate (Ba(HSO₄)₂).
Sulfate Ion (SO₄²⁻):
-
Weak Base: The sulfate ion is a very weak base. It has a negligible tendency to accept a proton. This implies it is not likely to undergo significant reactions involving proton acceptance.
-
Solubility: Sulfate salts show varying solubility. Some, such as sodium sulfate (Na₂SO₄) and potassium sulfate (K₂SO₄), are highly soluble in water; others, such as barium sulfate (BaSO₄) and lead sulfate (PbSO₄), are practically insoluble. This difference in solubility is crucial in analytical chemistry.
-
Complex Formation: The sulfate ion can form complexes with certain metal ions. These complexes often have unique properties that can be exploited in various applications.
Significance and Applications of the Conjugate Bases
Both HSO₄⁻ and SO₄²⁻ are important in various chemical applications:
Hydrogen Sulfate Ion (HSO₄⁻):
-
Acid Catalysts: HSO₄⁻ is used as an acid catalyst in many industrial processes, such as esterification and dehydration reactions. Its intermediate acidity is often desirable for controlling reaction rates.
-
Electrolyte in Batteries: Hydrogen sulfate salts can act as electrolytes in certain types of batteries, facilitating the flow of current.
-
Chemical Intermediates: HSO₄⁻ serves as a crucial intermediate in many chemical reactions, playing a vital role in synthesis and transformations.
Sulfate Ion (SO₄²⁻):
-
Fertilizers: Sulfate salts are essential components of many fertilizers, providing sulfur, a crucial nutrient for plant growth.
-
Industrial Processes: SO₄²⁻ plays a significant role in various industrial processes, including the production of chemicals, paper, and textiles.
-
Precipitations: The low solubility of certain sulfate salts (like BaSO₄) finds application in analytical chemistry as a means of separating or identifying certain metal ions through precipitation reactions.
-
Medical Imaging: Barium sulfate’s insolubility and radiopacity make it a crucial component of barium meals used in medical imaging of the gastrointestinal tract.
Conclusion: A Comprehensive Understanding
The conjugate bases of H₂SO₄, HSO₄⁻ and SO₄²⁻, are not merely byproducts of acid dissociation; they are chemically significant species with unique properties and diverse applications. Understanding their amphoteric nature, acid-base strengths, and solubility is critical to appreciating sulfuric acid's role in chemistry and related industries. From industrial catalysis to fertilizer production and medical imaging, these conjugate bases play pivotal roles, highlighting the importance of understanding acid-base chemistry at a molecular level. The information provided here offers a deep dive into this essential aspect of chemistry, emphasizing the significant influence of these conjugate bases on numerous chemical phenomena and practical applications. Further exploration into specific applications of HSO₄⁻ and SO₄²⁻ will reveal even more about their diverse roles in our world.
Latest Posts
Latest Posts
-
What Is The Most Reactive Element
Mar 21, 2025
-
What Is The Electron Configuration For Ne
Mar 21, 2025
-
Dna Replication Is Considered Semiconservative Because
Mar 21, 2025
-
Find Two Positive Real Numbers Whose Product Is A Maximum
Mar 21, 2025
-
How Many Inches In A 1 4 Yard
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about What Is The Conjugate Base Of H2so4 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
